Your cart is currently empty!
Level: Chapter
Chapter Level Content
Factorisation | Study
Introduction to Graphs | Study
MATTER IN OUR SURROUNDINGS | Study
IS MATTER AROUND US PURE | Study
ATOMS AND MOLECULES | Study
STRUCTURE OF THE ATOM | Study
THE FUNDAMENTAL UNIT OF LIFE | Study
TISSUES | Study
MOTION | Study
FORCE AND LAWS OF MOTION | Study
GRAVITATION | Study
WORK AND ENERGY | Study
SOUND | Study
STATISTICS | Study
SURFACE AREAS AND VOLUMES | Study
NUMBER SYSTEMS | Study
Chemical Reactions and Equations | Study
Acids, Bases and Salts | Study
Metals and Non-metals | Study
Carbon and its Compounds | Study
Life Processes | Study
How do Organisms Reproduce | Study
Heridity | Study
Control and Coordination | Study
Factorisation | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Factorisation: Representation of an algebraic expression as the product of two or more expressions is called factorization. Each such expression is called a factor of the given algebraic expression. (Scroll down till end of the page) Study Tools Audio, Visual & Digital Content When we factorise… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Factorisation: Representation of an algebraic expression as the product of two or more expressions is called factorization. Each such expression is called a factor of the given algebraic expression. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
When we factorise an expression, we write it as a product of factors. These factors may be numbers, algebraic variables or algebraic expressions.
An irreducible factor is a factor which cannot be expressed further as a product of factors.
A systematic way of factorising an expression is the common factor method. It consists of three steps:
- Write each term of the expression as a product of irreducible factors
- Look for and separate the common factors and
- Combine the remaining factors in each term in accordance with the distributive law.
Sometimes, all the terms in a given expression do not have a common factor; but the terms can be grouped in such a way that all the terms in each group have a common factor. When we do this, there emerges a common factor across all the groups leading to the required factorisation of the expression. This is the method of regrouping.
In factorisation by regrouping, we should remember that any regrouping (i.e., rearrangement) of the terms in the given expression may not lead to factorisation. We must observe the expression and come out with the desired regrouping by trial and error.
A number of expressions to be factorised are of the form or can be put into the form: a2 + 2ab + b2, a2 – 2ab + b2, a2 – b2 and x2 + (a + b)x + ab. These expressions can be easily factorised using Identities I, II, III and IV
a2 + 2ab + b2 = (a + b)2
a2 – 2ab + b2 = (a – b)2
a2 – b2 = (a + b) (a – b)
Factorisation
x2 + (a + b)x + ab = (x + a)(x + b)
In expressions which have factors of the type (x + a) (x + b), remember the numerical term gives ab.
Its factors, a and b, should be so chosen that their sum, with signs taken care of, is the coefficient of x.
We know that in the case of numbers, division is the inverse of multiplication. This idea is applicable also to the division of algebraic expressions.
In the case of division of a polynomial by a monomial, we may carry out the division either by dividing each term of the polynomial by the monomial or by the common factor method.
In the case of division of a polynomial by a polynomial, we cannot proceed by dividing each term in the dividend polynomial by the divisor polynomial. Instead, we factorise both the polynomials and cancel their common factors.
In the case of divisions of algebraic expressions that we studied in this chapter, we have Dividend = Divisor × Quotient.
In general, however, the relation is Dividend = Divisor × Quotient + Remainder
Thus, we have considered in the present chapter only those divisions in which the remainder is zero.
There are many errors students commonly make when solving algebra exercises.
You should avoid making such errors.
Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
Introduction to Graphs | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Graphical presentation of data is easier to understand. A bar graph is used to show comparison among categories. A pie graph is used to compare parts of a whole. A Histogram is a bar graph that shows data in intervals. (Scroll down till end of the… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Graphical presentation of data is easier to understand.
A bar graph is used to show comparison among categories.
A pie graph is used to compare parts of a whole.
A Histogram is a bar graph that shows data in intervals. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Introduction to Graphs
A line graph displays data that changes continuously over periods of time. A line graph which is a whole unbroken line is called a linear graph.
For fixing a point on the graph sheet we need, x-coordinate and y-coordinate.
The relation between dependent variable and
through a graph.
independent variable is shown
A Bar Graph: A pictorial representation of numerical data in the form of bars (rectangles) of uniform width with equal spacing. The length (or height) of each bar
represents the given number.
A Pie Graph: A pie graph is used to compare parts of a whole. The various
observations or components are represented by the sectors of the circle.
A Histogram: Histogram is a type of bar diagram, where the class intervals are shown on the horizontal axis and the heights of the bars (rectangles) show the frequency of the class interval, but there is no gap between the bars as there is no gap between the
class intervals.
Linear Graph: A line graph in which all the line segments form a part of a single line. Coordinates: A point in Cartesian plane is represented by an ordered pair of numbers.
Ordered Pair: A pair of numbers written in specified order.

Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
MATTER IN OUR SURROUNDINGS | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Matter: 1. Characteristics of Matter Particles Anything (Physical Material not emotions, feelings etc.) which has mass and volume (occupy space) is called matter. We feel the presence of matter by one or more of our five sense organs. Matter is made up of particles. (Scroll down… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Matter:
1. Characteristics of Matter Particles
Anything (Physical Material not emotions, feelings etc.) which has mass and volume (occupy space) is called matter.
We feel the presence of matter by one or more of our five sense organs.
Matter is made up of particles. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Particles:
Particles are very small in size. Therefore we cannot see particles with our naked eye.
Characteristics of the particles of matter:
(1) All matter (elements or compounds) consists of very small particles which can exist independently and are called particles.
(ii) The particles of matter are in a state of continuous motion and possess kinetic energy.
(iii) There are intermolecular spaces in between the particles (molecules) of matter.
(iv) The particles (molecules) of matter attract each other with a force called intermolecular force.
Intermolecular force is maximum in solids and least in the gases.
These material particles can be touched, moved by changing temperature or attracted by decreasing or increasing forces of attraction or repulsion.
2. States of Matter
Matter exists in three different physical states namely solid, liquid and gas.
One substance such as water can exist in all the three states such as, ice in solid state, water in liquid state and steam or vapours in gaseous state.
The state of matter depends on temperature, forces of attraction between their constituent particles etc.
3. Interconversion of Matter
All these three different states of matter are interconvertible depending upon temperature and pressure.
The state of matter can be changed by changing temperature or pressure.
Due to change in temperature and pressure there will be a change in inter-particle space as well as force between them, resulting in change in physical state.
Examples:
- Applying pressure and reducing temperature can liquefy gases.
- Solid CO₂ gets converted directly to a gaseous state on decrease of pressure to 1 atmosphere without changing into a liquid state. Due to this fact solid CO₂ is also known as DRY ICE.
4. Plasma: It is the fourth state of matter consisting of super energetic and super excited particles. These particles are in the form of ionised gases.
Examples:
- The plasma in stars is formed due to high temperature.
- Glowing plasma formed in fluorescent tubes and neon sign bulbs.
These devices contain inert gases which get ionised due to the passage of electric current. The colour of the glowing plasma depends upon the nature of the gas.
5. Sublimation: The process in which a solid state directly changes into a gaseous state on heating or vice-versa on cooling.
6. Melting or Fusion: The process of changing a solid into a liquid state by absorbing heat at a constant temperature is known as Melting or Fusion.
7. Freezing or Solidification: The process of changing a liquid into solid state by losing heat at a constant temperature is known as Freezing or Solidification.
8. Condensation: The process of changing a gas into a liquid state by giving out heat at constant temperature is known as Condensation .
Boiling or Vaporisation : The process of changing a liquid into a gaseous state by absorbing heat at constant temperature is known as Boiling or Vaporisation .
Boiling is a bulk phenomenon. Particles from the bulk (whole) of the liquid change into a vapour state.
Evaporation: The phenomenon of changing the physical state from liquid to vapour, at any temperature is called evaporation.
Evaporation is a surface phenomenon. Particles from the surface gain required energy to overcome the forces of attraction present in the liquid and change into the vapour state.
The rate of evaporation depends upon the surface area exposed to the atmosphere, the temperature, the humidity and the wind speed.
Evaporation causes cooling.
Evaporation takes place at all temperatures, below the boiling point of a liquid
Factors affecting evaporation:
• Rate of evaporation increases with increase in surface area.
• Rate of evaporation increases with increase in temperature.
• Rate of evaporation increases with decrease in Humidity.
• Rate of evaporation increases with increase in wind speed.
Latent heat of boiling or Latent heat of Vaporisation: Latent heat of boiling or Latent heat of Vaporisation is the heat energy required to change 1 kg of a liquid to gas at atmospheric pressure at its boiling point.
Kelvin is the SI unit of temperature.
0°C = 273.16 K.
For convenience, we take 0°C = 273 K after rounding off the decimal.
To change a temperature on the Kelvin scale to the Celsius scale you have to subtract 273 from the given temperature, and to convert a temperature on the Celsius scale to the Kelvin scale you have to add 273 to the given temperature.
Conversion Formula: t°C = (t+273) K
Boiling point or Vaporisation point: Boiling point or Vaporisation point is the fixed temperature at which a liquid converts into a gaseous state at atmospheric pressure.
Melting point or Fusion point: Melting point or Fusion point is the temperature at which a solid starts converting into a liquid state at atmospheric pressure.
Evaporation Causes cooling: During evaporation the particles at the surface of the liquid gain energy from the surroundings and change into vapour.. Therefore Evaporation Causes cooling effect.
Sponge can be compressed although it is solid: Sponge contains minute holes in which air is trapped.So when it is pressed, the air gets expelled and the sponge gets compressed. Also,the material of the sponge is not rigid.
Temperature does not change during change of state: The temperature remains constant at its melting and boiling points (during change of state) until all the substance melts or boils.
Because the heat supplied is continuously used up in changing the state of the substance by overcoming the force of attraction between the particles.
There is no increase in the kinetic energy of the particles and thus, temperature does not change.
This heat energy absorbed without showing any rise in temperature is given the name latent heat of fusion/latent heat of vaporisation.
Effect of pressure on physical state of a substance:
If pressure is applied, melting point decreases and boiling point increases
When pressure is increased, the particles come closer and the force of attraction increases between them and this results in a change of state.
Example: When high pressure is applied to a gas by reducing its temperature, the particles of gas come close and get converted to a liquid. This is also known as liquefaction.
The amount of heat energy required in changing a 1 kg of solid into liquid at atmospheric pressure and its melting point is known as the latent heat of fusion.
[ Lice = 80 cal/g = 3.34 × 105 J/kg].
• The amount of heat which is required to convert 1 kg of the liquid (at its boiling point) to vapours of gas without any change in temperature is known as latent heat of vaporisation.
[Lwater =540 cal/g= 22.5 × 105 J/kg].
• The amount of heat absorbed or liberated , Q = mL.
• The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
• Q = m.s. t, where m = mass of the body, s = specific heat of the body and t is temperature difference and m.s is called thermal capacity.
• Change of liquid into vapours at any temperature below the boiling point.
Takes the latent heat from the body. Thus, the body cools when evaporation takes place.
Evaporation:
(1) Evaporation is a slow process.
(ii) Evaporation takes place at the surface mass of the liquid.
(iii) Evaporation takes place at all temperatures.
(iv) The substance becomes cool due to evapora- tion process.
(v) Heat is absorbed from the surroundings due to Evaporation. Absorption of heat from the surroundings causes cooling effect.
Boiling:
(1) Boiling is a rapid process.
(ii) Boiling takes place throughout the mass of a liquid.
(iii) Boiling takes place at a definite temperature called the boil- ing point.
(iv) The substance remains hot during the boiling process.
(v) Heat is required from an external source such as a burner for boiling to take place.
Scales of temperature
• Three scales are commonly used for measuring temperature, namely, the Celsius scale, the Fahrenheit scale and the Kelvin scale.
• The relation between the Celsius and the Kelvin scale can be expressed as:
C + 273 = K
• The relation between the Celsius and the Fahrenheit scale can be expressed as follows.
Property Solid Liquid Gas Inter particle space Very less Larger than solid butlesser than gas Very large Inter particle force Very strong Weaker than solidbut stronger than gas Very weak Nature (Rigidity) Very hard and rigid Fluid Highly fluid Compressibility Negligible Very small Highly compressible Shape Definite shape Indefiniteshape Indefinite Shape Volume Definite Volume Indefinite shape Indefinite volume Density high Less than solid Very low Kinetic energy low high Very high Diffusion Negligible Slow Very high Specific Heat
11.8 NATURAL PHENOMENA AND CONSEQUENCES OF HIGH SPECIFIC HEAT CAPACITY OF WATER
Some consequences of high specific heat capacity of water are given below.
(i) The climate near the seashore is moderate :
The specific heat capacity of water is very high (= 1000 cal kg-1 °C-1 or 4200 J kg-1 K-¹). It is about five times as high as that of sand. Hence the heat energy required for the same rise in temperature by a certain mass of water will be nearly five times that required by the same mass of sand.
Similarly, a certain mass of water will give out nearly five times more heat energy than that given by sand of the same mass for the same fall in temperature.
As such, sand (or earth) gets heated or cooled more rapidly as compared to water under similar conditions.
Thus, a large difference in temperature is developed between the land and the sea due to which land and sea breezes are formed”. These breezes make the climate near the seashore moderate.
(ii) Hot water bottles are used for fomentation: The reason is that water does not cool quickly due to its large specific heat capacity, so a hot water bottle provides heat energy for fomentation for a long time.
(iii) Water is used as an effective coolant: By allowing water to flow in pipes around the heated parts of a machine, heat energy from such parts is removed (e.g. radiators in car and generator are filled with water). Water in pipes extracts more heat from surroundings without much rise in its temperature because of its large specific heat capacity.
(iv) In cold countries, water is used as a heat reservoir for wine and juice bottles to avoid their freezing: The reason is that water due to its high specific heat capacity can impart a large amount of heat before reaching up to the freezing temperature. Hence bottles kept in water remain warm and they do not freeze even when the surrounding temperature falls considerably.
(v) Farmers fill their fields with water to protect the crops from frost: In the absence of water, if on a cold winter night, the atmospheric temperature falls below 0°C, the water in the fine capillaries of plants will freeze, so the veins will burst due to the increase in volume of water on freezing. As a result, plants will die and the crop will be destroyed. In order to save crop on such cold nights, farmers fill their fields with water because water has a high specific heat capacity, so it does not allow the temperature in the surrounding area of plants to fall up to 0°C.
(vi) All plants and animals have a high content of water in their bodies: All plants and animals have nearly 80% to 90% of water in their bodies so it helps in maintaining the body temperature nearly same in all seasons due to high specific heat capacity of water.
SOME EXAMPLES OF HIGH AND LOW THERMAL CAPACITY
(1) The base of a cooking pan is made thick : By making the base of the cooking pan thick, its thermal capacity becomes large and it imparts sufficient heat energy at a low temperature to the food for its proper cooking. Further it keeps the food warm for a long time, after cooking.
(2) The base of an electric iron is made thick and heavy: By doing so, the thermal capacity of the base becomes large and it remains hot for a long duration even after switching off the current.
(3) The vessel used for measurement of heat (i.e., calorimeter) is made of thin sheet of copper:
The reason is that the specific heat capacity of copper is low and by making the vessel thin, its thermal capacity becomes low so that it takes a negligible amount of heat from its contents to attain the temperature of the contents.
Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
IS MATTER AROUND US PURE | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Matter: Anything that occupies space is called matter. Example: Air, water, rock etc., Matter exists in our surroundings in both pure and impure forms. (Scroll down till end of the page) Study Tools Audio, Visual & Digital Content Mixture: A mixture is a matter that contains… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Matter: Anything that occupies space is called matter.
Example: Air, water, rock etc.,
Matter exists in our surroundings in both pure and impure forms. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Mixture: A mixture is a matter that contains more than one pure substance in any ratio/proportion.
A mixture is an impure form of matter.
Example:
Water in milk, lemon juice, Ginger Garlic paste, etc.,
The mixture may or may not be separated into its constituent particles by physical processes.
Substance: A matter that cannot be separated into its constituent particles by any physical process is known as a substance.
Example:
Solution: A homogeneous mixture of two or more substances is called a solution.
Example:
Tea, sugar, and common salt are dissolved in water.
Alloy: A homogeneous mixture of metals is called an alloy.
Properties of the Solution:
- A solution is a homogeneous mixture
- Particles are extremely small, not visible to the naked eye
- The light path is invisible in solution.
- Solute particles cannot be separated by filtration
Concentration of solution: The concentration of a solution is the amount of solute present in a given quantity of the solution.
Unsaturated and Saturated Solutions: a solution in which a larger quantity of solute can be dissolved without raising its temperature, is called an unsaturated solution.
• A solution in which no more solute can be dissolved at a certain temperature, is called a saturated solution.
Solubility: The maximum amount of a solute that can be dissolved in 100 grams of a solvent at a specified temperature is known as the solubility of the solute in that solvent.
Suspension: a heterogeneous mixture of solids and liquids where the solid particles are suspended throughout the medium.
Example: Mixture of chalk powder and water
Properties of Suspension
• Particles are visible to the naked eye
• Light path in a suspension is visible
• Particles settle down
Colloidal Solution: Colloidal Solution Is a heterogeneous mixture, but appears to be homogeneous.
Examples: Milk, soap lather, soda water, pumice stone, rubber, bread, fog, cloud, insecticide spray, butter, etc.
Properties of colloidal solutions
• Heterogeneous mixture
• Particle size is small, not visible to the naked eye
• Light path can be visible;
• Particles do not settle down
• Substances cannot be separated by filtration
Tyndall Effect: Scattering of light beam by suspended particles in the solution.
Physical and Chemical changes:
Physical and change: The changes in which no new substances are formed are called physical changes.
Chemical change: The changes in which new substances are formed are called chemical changes.
SEPARATION OF MIXTURES
The method of separation depends on both the type of mixture and the physical properties of its constituents.
These are :
(i) The physical state of the constituents.
(ii) The differences in the physical properties
of the constituents, such as:
(a) boiling point
(b) melting point
(c) density
(d) magnetic properties
(e) ability to sublime
(f) volatility
(g) solubility in various solvents.
• Evaporation: Used for separating mixtures of volatile solvents and non-volatile solutes.
Working Principle:
One component should be non-volatile. It may or may not be soluble in water.
Example: Separating salt from its solution
• Centrifugation used for separating components based on the difference in their weights.
Working Principle:
Difference in the densities of two liquids.
Example: Separating mixtures of cream from milk
• Separating Funnel: Used for separating two or more immiscible liquids.
Working Principle:
Immiscible liquids with different densities get separated into different layers if they are in the same container.
Example: Separating oil and water
Sublimation:
Sublimation is the process of converting a solid into vapour and returning it to the solid state without passing through the liquid state.
Sublimation is used to separate sublimable solids from their mixtures.
Working Principle:
One of the components can be sublime.
Example: Separating ammonium chloride from a mixture
Chromatography:
The process of separating the different dissolved constituents of a mixture by their adsorption (adsorption refers to the collection of one substance on the surface of another substance.) over an appropriate adsorbing material is called chromatography.
Chromatography is used to separate those solutes that dissolve in the same solvent.
Working Principle:
Adsorption/partition
Example: Separating the components of a dye
Distillation:
Distillation is the process of heating a liquid to convert it into vapours and then condensing the vapours back into a liquid.
Distillation is used to separate two miscible liquids that boil without decomposition.
Working Principle:
One component should be a soluble solid in a liquid.
Example: Separating a mixture of acetone and water
Fractional distillation
Fractional distillation is a process that involves the distillation and collection of fractions or different liquids boiling at different temperatures.
Fractional distillation is used to separate a mixture of liquids when their boiling temperatures differ by less than 25 K.
Example: Separating different components of petroleum
Crystallization: Used to separate pure solids from a solution by forming crystals.
Working Principle:
A solid dissolved in a liquid is separated by evaporating the solvent completely by heating the mixture.
Example: Obtaining pure crystals of copper sulphate from an impure sample.
Differences Between Mixture And Compound
Property Mixture Compound Nature When two or more elements or compounds or both are mixed together, such that they do not combine chemically, a mixture is formed. When two or more elements unitechemically, a compound is formed. Structure Mixtures are generally heterogeneous. However, some mixtures can be homogeneous. Compounds are always homogeneous. Composition In case of mixtures their constituents can be present in any ratio, i.e., mixtures havevariable composition. In case of compounds, the constituents arepresent in a fixed ratio by weight. Properties The constituents of a mixture retain theirindividual chemical and physical properties. The properties of a compound are entirelydifferent from the properties of itsconstituents Separation of constituents The constituents of a mixture can beseparated by applying physical methods likesolubility, filtration, evaporation, distillation,use of magnet, etc. The constituents of a compound cannot beseparated by applying physical methods.However, constituents of a compound can beseparated by chemical means. Energy change There may or may not be energy changeduring the formation of mixture. During the formation of a compound eitherthe energy is absorbed or given out. Type of Mixture Nature of Mixture Example Separation Method Solid – solid Heterogeneous Iron + Sand; Magnetic separation Solid – solid Heterogeneous Iodine + Sand Sublimation Solid – solid Heterogeneous Iron + Sulphur Solvent extraction Solid – solid Heterogeneous Nitre + Common salt Fractional crystallisation Solid – liquid Heterogeneous Sand+Water; Clay + Water Sedimentation-decantation Solid – liquid Heterogeneous Chalk + Water; PbCl₂ + Water Filtration Solid – liquid Homogeneous Common salt in seawater Evaporation Solid – liquid Homogeneous Iodine + Methyl alcohol Distillation Liquid – liquid Homogeneous Methyl alcohol + Ethyl alcohol Fractional distillation Liquid – liquid Homogeneous Oil + Water; Mercury + Water Separating funnel Liquid – gas Homogeneous Ammonia + Water Boiling of liquid Complex Mixture Homogeneous Colouring matter in ink Chromatography Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
ATOMS AND MOLECULES | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Atoms are the basic building blocks of matter. Different kinds of matter contain different kinds of atoms present in them. Protons were discovered by Ernest Rutherford, in his famous gold foil experiment. Electrons were discovered by J.J. Thomson, in his cathode ray tube experiment. Neutrons were… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Atoms are the basic building blocks of matter.
Different kinds of matter contain different kinds of atoms present in them.
Protons were discovered by Ernest Rutherford, in his famous gold foil experiment.
Electrons were discovered by J.J. Thomson, in his cathode ray tube experiment.
Neutrons were discovered by James Chadwick. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Laws of Chemical Combination: Antoine Laurent Lavoisier, is known as ‘Father of Modern Chemistry.
Lavoisier put forward the law of conservation of mass, which laid the foundation of chemical sciences.
Law of Conservation of Mass: Law of Conservation of Mass states that, “mass is neither created nor destroyed in a chemical reaction.
In other words, the mass of the reactants must be equal to the mass of products.
Law of Constant Proportions or Definite Composition: Law of Constant Proportions or Definite Composition states that, in a pure chemical substance, the elements are always present in definite proportions by mass.
Dalton’s Atomic Theory:
(i) Every element is composed of extremely small particles called atoms.
(ii) Atoms of a given element are identical, both in mass and properties.
(iiii) Different chemical elements have different kinds of atoms; in particular, their atoms have different masses.
(iv) The atoms neither be created nor be destroyed or transformed into atoms of other elements.
(v) Compounds are formed when atoms of different elements combine with each other in small whole number ratios.
(vi) The relative number and kinds of atoms in a given compound are constant.
Drawbacks of Dalton’s Atomic Theory:
(i) According to modern theory, an atom is not the ultimate indivisible particle of matter. Today, we know that atoms are divisible, they are themselves made-up of particles (protons, electrons, neutrons,etc.).
(i) In the case of isotopes of an element, the assumption that the atoms of the same element have the same mass does not hold good.
Atom: It is the smallest particle of an element that maintains its chemical identity throughout all chemical and physical changes.
The smallest unit of a substance which can exist independently is called a molecule.
Atomicity: It is defined as the number of atoms present in a molecule of an element or a compound.
Mono atomic: Molecule having only one atom is called mono atomic,
e.g., He, Ne, Ar.
Diatomic: Molecules made-up of two atoms are called diatomic, e.g., H₂, Cl₂, O₂, N2.
Triatomic: Molecules made-up of three atoms, called triatomic.
e.g., O3, H₂O, NO2.
Tetraatomic : Molecules made-up of four atoms, called tetra atomic.
e.g., P4, NH3, SO3
Polyatomic: Molecules made-up of five or more atoms, called polyatomic/
e.g., CH4.
Polyatomic: Any molecule which is made-up of more than four atoms is called polyatomic,
e.g., Sg.
Relative Atomic Mass: It is defined as the number of times one atom of an element is heavier than
(1/12)th of the mass of an atom of Carbon – 12.
Relative Atomic Mass (RAM) = Mass of an atom of an element/
¹/12 th mass of C-12
Molecular Mass: The molecular mass of a substance is the sum of the atomic masses of all atoms in a molecule of a substance,
e.g., molecular mass of water is 18 u.
The mole (or mol) is the SI unit of the amount of a substance. One mole is equal to the amount of substance that contains as many elementary units as there are atoms in 12 g of the carbon-12 isotope.
The elementary units may be atoms, molecules, ions, radicals, electrons, etc., and must be specified.
This number is called Avogadro’s number (No) or Avogadro’s constant
[NA = 6.0221367 x 1023]. Generally,
Avogadro’s Number is rounded to 6.022 x 1023.
For better understanding we can compare avogadro number with a dozen as:
One dozen oranges contain 12 oranges, similarly, 1 mole of hydrogen atoms contain 6.022 x 1023 H atoms.
H₂O = 2 x H + 1 × O
= 2 x 1+1 x 16 = 2+16
= 18 amu or u.
By : 1 mole of a compound has a mass equal to its relative molecular mass expressed in grams.
1 mole = 6.022 × 1023 number
= Relative mass in grams.
A molecule is the smallest particle of an element or a compound capable of independent existence under ordinary conditions. It shows all the properties of the substance.
A chemical formula of a compound shows its constituent elements and the number of atoms of each combining element.
Clusters of atoms that act as an ion are called polyatomic ions. They carry a fixed charge on them.
The chemical formula of a molecular compound is determined by the valency of each element.
In ionic compounds, the charge on each ion is used to determine the chemical formula of the compound.
Scientists use the relative atomic mass scale to compare the masses of different atoms of elements. Atoms of carbon-12 isotopes are assigned a relative atomic mass of 12 and the relative masses of all other atoms are obtained by comparison with the mass of a carbon-12 atom.
The Avogadro constant 6.022 × 1023 is defined as the number of atoms in exactly 12 g of carbon-12.
The mole is the amount of substance that contains the same number of particles (atoms/ions/ molecules/formula units, etc.) as there are atoms in exactly 12g of carbon-12. Mass of 1 mole of a substance is called its molar mass.
The relative atomic mass of the atom of an element is the average mass of the atom as compared to 1/12th mass of one carbon-12 atom.
Hint: We know that chemical formulas can also be written using a criss-cross method. In the criss-cross method, the numerical value of the ion charge of the two atoms is crossed over, which becomes the subscript of the other ion. Using this technique, we will write the chemical formula of the given compounds.
Complete step by step answer:
Let’s us discuss about the given compound as,
A.Magnesium chloride
We have to remember that the atomic number of Magnesium is 12 and has a valency of 2.
It means it has two electrons in the outermost shell for bonding.
The atomic number of chlorine is 17 and has 7 electrons in the outermost shell.
It means it just needs one more atom for bonding.
Hence, we will use atoms of chlorine to bond with one atom of magnesium.
We can apply the criss-cross method for this compound as,
Therefore, the chemical formula of magnesium chloride is MgCl2
B.Calcium oxide
We have to know that the atomic number of calcium is 20 and has a valency of 2, it means it has 2 two atoms in the outermost shell for bonding.
The atomic number of Oxygen is 8
8 and has a valency of 2, it has 6 atoms in the outermost shell, it needs 2 more to complete the octet.
Hence, we need one calcium atom to bond with one oxygen atom.
We can apply the criss-cross method for this compound as,
Therefore, the chemical formula of magnesium chloride is CaO
C. Copper nitrate
We have to know that the atomic number of copper is 29 and has two atoms in the outermost shell for bonding. While a nitrate molecule has only one valence electron.
We need 2 nitrate molecules to satisfy the valency of 1 copper atom.
We can apply the criss-cross method for this compound as,
Therefore, the chemical formula of magnesium chloride is
Cu(NO3)2
D.Aluminium chloride
We have to know that the atomic number of aluminium is 13 and has a valency of 3 atoms and chlorine atom has a valency of 1. Since it has 7 electrons in the outermost shell.
Thus, we need 3 chlorine atoms to satisfy the valency of 1 aluminium atom.
We can apply the criss-cross method for this compound as,
Therefore, the chemical formula of magnesium chloride is AlCl3.
E.Potassium nitrate
We have to remember that the atomic number of potassium is 19 and has a valency of 1 and nitrate also has a valency of 1, since it needs one more atom to complete its octet. Hence, we need only one molecule of nitrate for one atom of potassium.
We can apply the criss-cross method for this compound as,
Therefore, the chemical formula of magnesium chloride is KNO3.
Note: As we know that the criss-cross method is the most efficient way to write the correct chemical formula of the molecule. It is generally used for finding out the formula of a bonding of a metal with a non-metal to form ionic bonds. Signs of the two ions are dropped, the ion value is crossed which becomes the subscript of the crossed atoms.
Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
STRUCTURE OF THE ATOM | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Atoms are the basic building blocks of matter. Different kinds of matter contain different kinds of atoms present in them. Protons were discovered by Ernest Rutherford, in his famous gold foil experiment. Electrons were discovered by J.J. Thomson, in his cathode ray tube experiment. Neutrons were… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Atoms are the basic building blocks of matter.
Different kinds of matter contain different kinds of atoms present in them.
Protons were discovered by Ernest Rutherford, in his famous gold foil experiment.
Electrons were discovered by J.J. Thomson, in his cathode ray tube experiment.
Neutrons were discovered by James Chadwick. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Charged Particles in Matter
- Whenever we rub two objects together, they become electrically charged.
- This is because atoms contain charged particles in them.
- Therefore, atoms can be divided further into particles i.e proton, electron and neutron.
Atoms consist of an equal number of protons and electrons.
Protons exist in the interiors of the atom and electrons exist in the exteriors of the atom. Therefore, electrons can be removed from an atom.
Since electrons exist in the exteriors of the atom they can be removed from an atom.
Dalton’s Atomic Theory
The postulates of the atomic theory by John Dalton
- The matter is made up of tiny particles called Atoms that cannot be divided.
- Atoms are never formed or destroyed during a chemical reaction.
- Atoms of an element exhibit the same nature.
- Atoms of the same element have equal size, mass and they exhibit similar chemical properties.
- Atoms of different elements exhibit variant chemical properties.
- Atoms form compounds by combining in a ratio of whole numbers.
- A compound contains molecules in which a constant number and types of atoms are present.
Failure of Dalton’s Atomic Theory
Dalton suggested that atoms can neither be created nor destroyed and are indivisible.
But the discovery of electrons and protons in atoms disproved this aspect of Dalton’s theory.
Thomson’s Model of an Atom
According to J.J. Thomson, the structure of an atom can be compared to Christmas pudding.
According to this model the electrons are present inside a positive sphere.
An atom is composed of a positively charged sphere in which electrons are embedded.
Atoms are neutral as the positive and negative charges are equal in number.
Rutherford’s Model of an Atom
Rutherford’s Experiment
Rutherford experimented by passing alpha rays through a thin gold foil.
He expected that the gold atoms would deflect the Alpha particles.
Observations Inferences Alpha particles which had high speed moved straight through the gold foil Atom contains a lot of empty space Some particles got diverted a by small angles Positive charges in the atom are not occupying much of its space Only one out of 12000 particles bounced back The positive charges are concentrated over a particular area of the atom. Based on his experiment Rutherford gave the nuclear model of an atom as the following.
Rutherford’s Atomic Model
Rutherford’s Atomic Model is known as Planetary Atomic Model and Nuclear Atomic Model.
According to Rutherford’s Atomic Model:
- Atoms contain a lot of unoccupied space
- The center of the atom is highly positive , Rutherford named it as nucleus
- The atom contains an equal amount of positive and negative charges.
Nucleus of Atom
The nucleus is located at the center of the atom.
All the mass of the atom is because of the nucleus.
The electrons revolve around the nucleus in circular parts which called Orbits
The size of an atomic nucleus is much smaller than its atom.
Drawbacks of the Nuclear Atomic Model
The Rutherford’s Atomic Model failed to explain how an atom remains stable despite having positive and negative charges present in it.
Maxwell’s theory of radiation if any charged particle moves in a circular motion it radiates energy.
So, if electrons move in a circular motion around the nucleus they should radiate some energy as a result this decreases at the speed of the electrons. As a result, they would fall into the nucleus and the nucleus should collapse because of its high positive charge.
But it is not happening because the matter is not collapsing.
Nucleons: The subatomic particles present in the nucleus are collectively called Nucleons. Protons and Neutrons are nucleons.
Bohr’s Model of an Atom
Bohr Atomic Model states as the following:
- Electrons revolve around the nucleus in particular circular paths, called orbits.
- The electrons do not emit any energy while moving in their orbits.
- The orbits are also called Energy Levels.
- Energy Levels or Orbits are represented by using letters or numbers as shown in the figure.
Neutron:
J. Chadwick discovered Neutron, a subatomic particle of an atom.
Neutron carries no charge.
Subatomic Particles of Atom
Electrons Electron carry a negative charge Protons Protons carry a positive charge Neutrons Neutrons are neutral Electronic Configuration: The distribution of electrons in different shells or orbits is called Electronic Configuration.
- If Orbit number = n
- Then number of electrons present in an Orbit = 2n2
- So, for n =1
- Maximum electrons present in shell – K = 2 * (1)2 = 2
- The outermost shell can contain at most 8 electrons.
- The shells in an atom are filled in sequence.
- Thus, until the inner shells of an atom are filled completely the outer shells cannot contain any electrons.
Valency
- Valence Electrons – Electrons existing in the outermost orbit of an atom are called Valence Electrons.
- The atoms which have completely filled the outermost shell are not very active chemically.
- The valency of an atom or the combining capacity of an atom is given by the number of elements present in the outermost shell.
- For Example, Helium contains two electrons in its outermost shell which means its valency is two. In other words, it can share two electrons to form a chemical bond with another element.
- What happens when the outermost shell contains a number of electrons that are close to its maximum capacity?
Valency in such cases is generated by subtracting the number of electrons present in the outermost orbit from octet (8). For example, oxygen contains 6 electrons in its outermost shell. Its valency is calculated as: 8 – 6 = 2. This means oxygen needs two electrons to form a bond with another element.
Representation Element:
Atomic Number of an Element
Atomic Number (Z) = Number of protons in an atom
Mass Number of an Element
Mass Number = Number of protons + Number of neutrons
Isotopes
- The atoms of an element can exist in several forms having similar atomic numbers but varying mass numbers.
- Isotopes are pure substances.
- Isotopes have a similar chemical nature.
- Isotopes have distinct physical characteristics.
Use of Isotopes:
1. The fuel of Nuclear Reactor – Isotope of Uranium
2. Treatment of Cancer – Isotope of Cobalt
3. Treatment of Goiter – Isotope of Iodine
Example: Consider two atomic species namely U and V. Are they isotopes?
U V Protons 5 5 Neutrons 5 6 Mass Number 5 + 5 = 10 5 + 6 = 11 Atomic Number 5 5 From the above example, we can infer that U and V are isotopes because their atomic number is the same.
Isobars
The atoms of several elements can have a similar mass number but distinct atomic masses. Such elements are called Isobars.
Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
THE FUNDAMENTAL UNIT OF LIFE | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage What are Living organisms made up of?All living organisms are made up of cells. Cell is the basic structural and functional unit of complex organisms. History of cell: Cells were first discovered by Robert Hooke in 1665 with the help of a primitive microscope. Leeuwenhoek, in… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
What are Living organisms made up of?
All living organisms are made up of cells. Cell is the basic structural and functional unit of complex organisms.History of cell:
Cells were first discovered by Robert Hooke in 1665 with the help of a primitive microscope. Leeuwenhoek, in 1674, with the improved microscope, discovered free-living cells in pond water for the first time. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Robert Brown in 1831 discovered the nucleus in the cell.
Purkinje in 1839 coined the term ‘protoplasm‘ for the fluid part of the cell.
Schleiden in 1838 and Schwann in 1839 proposed the cell theory which stated that all plants and animals are composed of cells.
Rudolf Virchow in 1855 further expanded the cell theory by suggesting that all cells arise from pre-existing cells.
The invention of magnifying lenses led to the discovery of the microscopic world.
Unicellular organisms are the organisms in which a single cell performs all the functions like nutrition, respiration, excretion and reproduction.
Example: Amoeba, Chlamydomonas, Paramecium and Bacteria possess single cells constituting the whole organism. Multicellular organisms are the organisms which possess many cells to perform different functions.
Multicellular organisms represent themselves as a member of a group of cells or as an individual.
individual.
Example: Fungi, plants and animals have many cells that group together to form tissues.
Every multi cellular organism has come from a single cell. All cells thus come from pre existing cell.
Some organisms can also have cells of different kinds.

The shape and size of cell are related to the specific function they perform.
Some cells change their shapes.
Example: Amoeba. In some cases the cell shape could be more or less fixed and the peculiar for a particular type of cell.
Example: Nerve cells.
Each living cell has the capacity to perform certain basic functions that are characteristic of all living forms.There is a division of labour in multicellular organism such as human beings.
This means that different parts of the human body perform different functions.
Similarly division of labour is also seen within a single cell. In fact each such cell has got certain specific components
within it known as cell organelles. Each kind of cell organelle performs a special function.A cell is able to live and perform all its functions because of these organelles.
These organelles together constitute the basic unit called the cell. What is a cell made up of? What is the structural organization of a cell?
Every cell would have three features- plasma membrane, nucleus and cytoplasm.All activities inside the cell and interactions of the cell with its environment are possible due to these features. Plasma membrane or cell membrane:
This is the outermost covering of the cell that separates the contents of the cell from its external
environment. It is flexible and made up of organic molecules called lipids and proteins.The flexibility of the cell membrane also enables the cell to engulf in food and other material from its external environment. Such processes are known as endocytosis.
Example: Amoeba It allows the movement of some substances into and out of the cell.
It also prevents movement of
some other materials.Therefore it is called a selectively permeable membrane. Movement of substances through this semi-permeable membrane can be by the process of diffusion, osmosis etc.
Difference between diffusion and osmosis

If we put an animal cell or a plant cell into a hypotonic solution the cell is likely to swell up.
The cell will stay in the same size if it kept it in isotonic solution.
If the solution is hypertonic then the cell will shrink. Unicellular fresh water organism and most plants tend to gain water through osmosis.
Cell wall: It is present only in plant cells. The cell wall is composed of cellulose and is permeable. It
separates the contents of the cell from the surroundings. It gives shape and protection to the cell. Cell walls permit the cells of plants, fungi and bacteria to withstand very dilute external media without bursting.Plasmolysis: It is the process in which cells lose water in a hypertonic solution.
Nucleus:
The nucleus has a double layered covering called nuclear membrane. The nuclear membrane has
pores which allow the transfer of material from inside to outside. The nucleus contains
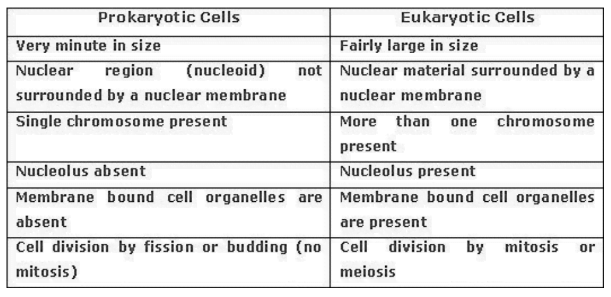
chromosomes which are composed of Deoxyribonucleic acid (DNA) and proteins. Nucleus
controls all the activities of the cell. As the nucleus carries genetic information in the form of DNA, it plays a major role in cell division and cell development. The functional segments of DNA are called genes. Nucleus plays
an important role in protein synthesis and transmission of characters from one generation to
another generation. It plays a central role in cellular reproduction. In some organisms nuclear
membrane is absent and nuclear region contains only nucleic acids called nucleoid. Such
organisms called prokaryotes. Eg. Bacteria. are called eukaryotes. Organisms with cells having a nuclear membrane
Cytoplasm:
The cytoplasm is the fluid content inside the plasma membrane. It is a jelly like viscous substance occupying entire cell except the nucleus. It also contains many specialized cell organelles that perform a specific function for the cell.
Cell organelles:
Cell organelles include endoplasmic reticulum, Ribosomes, Golgi apparatus, Mitochondria, Plastids, Lysosomes, and Vacuoles. They are important because they carry out some very crucial functions in cells.Endoplasmic reticulum (ER):
The ER is a large network of membrane bound tubes and sheets. It serves as channels for the transport of materials especially proteins between various organs of the cytoplasm or between the cytoplasm and nucleus. It also functions as a cytoplasmic framework providing a surface for some of the biochemical activities of the cell. There are two types of ER- Rough endoplasmic reticulum and smooth endoplasmic reticulum.RER: These are rough at surface and are associated with ribosomes. These are responsible for the synthesis of proteins. SER: These are smooth at surface and are not associated with ribosomes. It helps in the manufacture of fat molecules or lipids. It also plays a crucial role in detoxifying many poisons and drugs.
Membrane biogenesis: Some of the proteins and lipids synthesized by EF help in building the cell membrane. This process is known as membrane biogenesis.
Golgi Apparatus:
These cell organelles are named after the biologist, Camillo Golgi, who first described it. The Golgi consists of a stack of membrane-bound cisternae. These membranes often have connections with the membranes of ER and therefore constitute another portion of a complex cellular membrane system. Its functions include the storage, modification and packaging of products in vesicles. It is also involved in the formation of lysosomes.Lysososmes:
Lysosomes are membranous sacs filled with enzymes. These enzymes are made by RER. They are a kind of waste disposal system of the cell. They help to keep the cell clean by digesting any foreign material as well as worn out cell organelles. Lysosomes contain hydrolytic enzymes which are capable of digesting cellular macromolecules. When the cell gets damaged, the lysosome may burst and its enzymes may digest thecell itself. Hence, lysosomes are called as
‘suicidal bags’.Mitochondria:
These are cellular organelles termed as ‘power houses of the cells’. These are bounded by a double membrane. The outer membrane is smooth while the inner membrane is thrown into folds called as cristae. The cristae increase the area of cellular respiration. Mitochondria releases energy in the form of ATP molecules. ATP is known as the “energy currency of the cell”. Mitochondria have its own DNA DNA ribosomes and are able to make some of their own proteins.Plastids:
Plastids are present only in plant cells. These are of two types- chromoplasts (coloured plastids) and leucoplasts (white or colourless plastids). Plastid contains pigment called chlorophyll are known as chloroplasts. These are important for photosynthesis in plants. Chromoplasts are the organelles which provide bright colours to the plant structures like buds, flowers etc.
Leucoplasts: are the organelles which store starch, oils and protein granules. Plastids consist of numerous
membrane layers embedded in a material called the stroma. Plastids also have their own DNA
and ribosomes.Vacuoles: Vacuoles are membrane bound compartments present in both plant and animal cells. These are
storage sacs for solid or liquid contents. These are small sized in animal cells while bigger in plant cell. In plant cells vacuoles are full of sap and provide turgidity and rigidity to the cell. These organelles store water, waste products, and substances like amino acids, sugars and proteins. In some unicellular organisms specialized vacuoles also play important roles in expelling excess water and some wastes from the cell. Difference between plant cells and animal cellsDifference between Plant cells and Animal cells.

Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
TISSUES | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Are plants and Animals made of same types of tissues?Plants are stationary, and hence are provided with some tissues made up of dead cells, which provide mechanical strength. They have to withstand unfavourable conditions like strong winds, storms, floods etc. Animals on other hand move around… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Are plants and Animals made of same types of tissues?
Plants are stationary, and hence are provided with some tissues made up of dead cells, which provide mechanical strength. They have to withstand unfavourable conditions like strong winds, storms, floods etc. Animals on other hand move around in search of food, mates, shelter. (Scroll down till end of the page)Study Tools
Audio, Visual & Digital Content
They consume more energy as compared to plants. Most of the tissues they contain are living.
Cell growth in animas is more uniform.
The structural organisation of organs and organ systems is far more specialized and localised in complex animals than even in very complex plants.
Plant tissues:
Meristematic Tissue: The growth of plants occurs only in certain specific regions. This is because the dividing tissue
also known as meristematic tissue is the region where they are present, meristematic tissues are classified as apical, lateral and intercalary. Apical meristem is present at the apical or growing tips of stems and roots. Apical meristem
increases the length of the plant. Lateral meristem is present in the radial portion of the stem or root. Lateral meristem increases the girth of the plant.Intercalary meristem occurs at the base of the leaves or at the internodes. Intercalary meristem increases the length of the internode. Permanent Tissue Old meristematic cells lose the capacity to divide and transform into permanent tissues.
This process of taking up a permanent shape, size, and function is called differentiation. These are cells which have lost their capacity to divide but are specified to provide strength, flexibility and elasticity to the plant. These tissues can be further classified into simple permanent, complex permanent and special tissues. Simple permanent can be categorized into parenchyma, collenchyma and sclerenchyma based on their function. Parenchyma- they are live cells. They are usually loosely packed. This tissue provides support to plants and also stores food. In some situations it contains chlorophyll and performs photosynthesis and then it is called chlorenchyma. Parenchyma which contains large air cavities in aquatic plants is called aerenchyma. The aerenchyma helps in buoyancy. Collenchyma – These are elongated living cells with small intercellular spaces. Their cell walls
are made up of cellulose and pectin. Collenchyma occurs in the peripheral regions of stems and
leaves to provide mechanical support and flexibility in plants. Sclerenchyma – These are long, dead cells with a deposit of lignin in their cell wall. They have no intercellular spaces. Sclerenchyma occurs around the vascular tissues in stems, in the veins of leaves, and in the hard covering of seeds and nuts. They provide strength to the plant.Epidermis aids in protection against loss of water, mechanical injury and invasion by parasitic
fungi. Since it has a protective role to play, cells of epidermal tissue form a continuous layer
without intercellular spaces. Epidermis of the leaf contains small pores called stomata. These are
necessary for gases exchange and transpiration. Cork – This is the outer protective tissue which replaces the epidermal cells in older roots and stems. Cork cells are dead and lack intercellular spaces. Their cell walls are thickened by suberin
which makes them impermeable to water and gas molecules.Complex permanent tissue:
Complex permanent tissue comprises of conducting tissues called xylem and phloem. Xylem is useful in transport of water and soluble substances. Xylem consists of tracheids, vessels, fibres and xylem parenchyma. Transport of minerals and water is unidirectional in xylem. Phloem is useful in transport of food molecules. Phloem comprises of sieve tubes, sieve cells, companion cells, phloem fibres and phloem parenchyma. Phloem is unlike xylem in that materials can move in both directions in it.Animal Tissues:
These are the tissues present only in animals. Different types of animal tissues are epithelial tissue, connective tissue, muscle tissue and nervous tissue.
Epithelial Tissue:
Epithelial tissue forms a lining all over the body of the organism. It protects the inner lying
parts.It is also secretory in function to secrete sebum and excrete wastes along with sweat.
Sometimes it is absorptive in nature. Epithelial tissues act like a barrier to keep the different body systems separate. These are tightly packed and form a continuous sheet without intercellular spaces.
Squamous epithelium has flat and thin cells with no intercellular spaces.
Squamous epithelium provides is found in the outer layer of the skin, lining the cavities of blood vessels, lung alveoli, lining of oesophagus and the lining of the mouth. Stratified epithelium has epithelial cells lined up one over another. It is found in the epidermis of the skin.
It helps to prevent wear and tear of tissue. Columnar epithelium consists of cylindrical cells. It is found in the lining of the stomach and intestines, and facilitates the movement across the epithelial barrier.
Columnar epithelial tissue with cilia is known as ciliated epithelium. These cilia push the mucus forward into the nasal tract to clear it. Cuboidal epithelium consists of cubical cells. It is found in the lining of the kidney tubules, salivary glands and thyroid glands, where it provides mechanical support. Glandular epithelium consists of modified columnar cells, and is found in the sweat glands and tear glands to produce secretions.
Connective tissue :
Connective tissues are fibrous in nature.They include blood, bone, ligament, cartilage, areolar and adipose tissues.
These help in binding other tissues together. They also provide support to other tissues.
Blood has plasma and blood cells.
The blood cells suspended in the plasma include RBC’s, WBC’s and platelets.
Blood flows within blood vessels, and transports gases, digested food, hormones and waste materials to different parts of the body. Bone cells are embedded in a hard matrix composed of calcium and phosphorus compounds.
Bones anchor the muscles and support the main organs of the body. Two bones can be connected to each other by another type of connective tissue called ligament. Ligaments are tough and elastic. They provide strength and flexibility. Tendons connect muscles to bones and are another type of connective tissue. Tendons are tough and non-elastic, and provide great strength and limited flexibility. Cartilage has widely spaced cells suspended in a matrix of proteins and sugars. It is found in the nose, ears, and the rings of the trachea to give flexibility. Areolar connective tissue is found between the skin and muscles, around blood vessels and nerves
and in the bone marrow. It helps in repair of tissues. Adipose tissue contains cells filled with fat globules. It is found below the skin and acts as an
insulator.Muscular Tissue:
Muscle tissues consists of elongated cells also called muscle fibres.This tissue is responsible for movement.
Muscles contain special proteins called contractile proteins which contract and relax to cause movement.
These are elastic in nature they have tensile strength.
These muscles can be
voluntary or involuntary in function. Muscular tissues are of three kinds namely striated muscles, unstriated muscles and cardiac muscles. Striated muscle cells are long, cylindrical, unbranched and multinucleate.These are voluntary muscles.
Smooth muscles or involuntary muscles are found in the iris of the eye, in ureters and in the bronchi of the lungs.
These are also called unstriated muscles. The cells are long with pointed ends and uninucleate.
Hear muscles or cardiac muscles are cylindrical, branched and uninucleate.
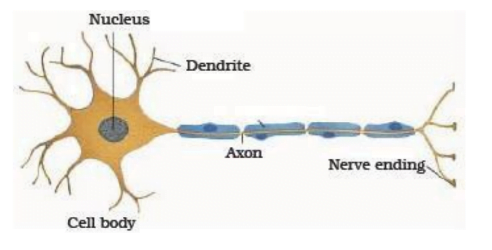
Nervous Tissue
Nervous tissues are found in the brain, spinal cord and nerves.Nervous tissue is the tissue which works in coordinating the organs of the body by generating impulses.
It is made up of special cells called as neurons.
Each neuron consists of a cell body, which contains a nucleus, cytoplasm, called cyton, from which long thin hair like parts arise.
Usually each neuron has a single long part, called the axon, and many short branched parts called dendrites.

Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
MOTION | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Rest And Motion The terms Rest and Motion are relative. Motion: An object is said to be in motion when its position changes with time. Rest: An object is said to be at rest when its position does not change with respect to a reference point… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Rest And Motion
The terms Rest and Motion are relative.
Motion: An object is said to be in motion when its position changes with time.
Rest: An object is said to be at rest when its position does not change with respect to a reference point with time.
A specific point with respect to which we describe the location of an object is called a reference point. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Distance and Displacement
- Distance: The total length of path covered by an object is said to be the distance travelled by it.
- Displacement: Gap between the initial and final positions of an object is said to be its displacement. Or
- The length of a line segment that joins the initial and final positions of an object is known as the displacement.
Difference Between Displacement and Displacement
Distance Displacement Distance is defined as the total length of the path travelled by an object to go from one point to another. Displacement is defined as the length of the line segment that joins the initial and final positions of an object. Since distance has only magnitude and its direction cannot be specified always, it is a scalar quantity. Since displacement has magnitude and it is specified in a direction from initial position to final position, it is a vector quantity. Distance can only have positive values. Displacement can have both positive and negative values. Distance depends on the length of the path travelled. Displacement depends only on the initial and final point regardless of the path travelled. Difference Between Displacement and Displacement Speed And Velocity Speed
- Speed: The distance travelled by an object in unit time is referred to as speed.
- Its S.I unit is m/s.
- In general speed refers to average speed.
- Average speed: For non-uniform motion, the average speed of an object is obtained by dividing the total distance travelled by an object by the total time taken.
- For a uniform motion, the average speed of an object is equal to its instantaneous speed throughout the path.
Velocity
- Average Velocity or Velocity : For a uniform motion in a straight path, the average velocity is equal to its instantaneous velocity throughout the path.
- Velocity of an object is equal to the instantaneous velocity of an object.
Differences Between Speed and Velocity
SPEED VELOCITY It is defined as the rate of change of distance. It is defined as the rate of change of net displacement. It is a scalar quantity. It is a vector quantity. It can never be negative or zero. It can be negative,zero or positive. Speed is velocity without direction. Velocity is directed speed. Speed may or may not be equal to velocity. A body may possess different velocities but the same speed. Speed never decreases with time. For a moving body, Velocity can decrease with time. For a moving body , it can be zero. Speed is never zero. Velocity can be zero. Speed in SI is measured in ms-1 Velocity in SI, is measured in ms-1 Differences Between Speed and Velocity Uniform And Non-Uniform motion
- Uniform motion or non accelerated motion: When an object covers equal distances in equal intervals of time, it is said to be in uniform motion. Uniform motion is a non-accelerated motion.
- Non-uniform motion or accelerated motion: Motions where objects cover unequal distances in equal intervals of time. Uniform motion is an accelerated motion.
Acceleration
Acceleration: Change in the velocity of an object per unit time.
Graphical representation of motions
(i) Distance-time graph
For a distance-time graph, time is taken on x-axis and distance is taken on the y-axis.
[Note: All independent quantities are taken along the x-axis and dependent quantities are taken along the y-axis.]
(ii) Velocity-time graph
Equation of motion by graphical methods
Derivation Of Equations Of Motion
Equations of motion can be derived by two methods. They are (i) Graphical Method. (ii) Algebraic Method
Derivation of The Equations of Motion By Algebraic Method:
(a) Velocity-time relation:
Derivation of S = ut + ½ at2
(ii) The equation for position-time relation:
Derivation of v2 – u2 = 2as
(iii) Equation for position-velocity relation:
Conclusions From a Distance – Time Graph
Uniform Circular Motion
When a body moves in a circular path with uniform speed, its motion is called uniform circular motion.
Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
FORCE AND LAWS OF MOTION | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Force : Push or pull is called Force. Example: We push or pull to open a door. (Scroll down till end of the page) Study Tools Audio, Visual & Digital Content Effects of Force Net or Resultant Force: Resultant Force or Net Force acts on a… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Force : Push or pull is called Force.
Example:
We push or pull to open a door. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Effects of Force
- Force can change the shape and size of an object.
- Force can move a stationary object.
- Force can change the speed of a body.
- Force can stop a moving body.
- Force can change the direction of a moving object.
Net or Resultant Force:
Resultant Force or Net Force acts on a body if two or more forces act on it at the same time. Resultant Force or Net Force on a body is defined as the net effective force due to the multiple forces acting on it simultaneously.
Based on Net force, Forces are classified into two types as:
(A) Balanced forces
(B) Unbalanced forces
(A) Balanced Forces
• If the resultant of applied forces is equal to zero, the forces are called balanced forces.
• Balanced forces do not cause any change in state of an object.
• Balanced forces can change the shape and size of an object.
For example, when forces are applied from both sides over a balloon, the size and shape of the balloon is changed.
(B) Unbalanced Forces
• If the resultant of applied forces are greater than zero, the forces are called unbalanced forces.
• Unbalanced forces can do the following :
* Move a stationary object
* Increase the speed of a moving object
* Decrease the speed of a moving object
* Stop a moving object
* Change the shape and size of an object
Laws of Motion :
Galileo Galilei :
Galileo Galilei was the first to say that objects move with a constant speed when no forces act on them.
That is, if there is no unbalanced force acting on the object, the object moves forever with a constant speed without changing its direction.
In other words, if an object is moving on a frictionless path and no other force is acting upon it, the object moves forever with a constant speed without changing its direction.
Galileo’s Experiment:
Galileo’s thought experiment considered rolling balls on inclined planes in the absence of friction or other resistant forces.
Galileo arranged two inclined planes opposite to each other as shown.
He rolls down the ball from the first inclined plane to climb the second inclined plane.
Galileo observations:
Galileo observed that:
- The ball rolling down the first inclined plane comes to rest after climbing a certain height on the second inclined plane.
- The speed acquired by the ball moving down a plane from a height is sufficient to enable it to reach the same height when climbing up another plane at a different inclination .
- As the angle decreases, the body should travel a greater distance.
From these observations, Galileo hypothesized as:
- if the force acting on the ball is only gravitational force, the height reached by the ball must be equal to the height from which it was rolled.
- When the inclinations of the two planes are the same, the distance travelled by the sphere while rolling down is equal to the distance travelled by it while climbing up.
- Now, if the inclination of the second plane is decreased slowly, then the sphere needs to travel over longer distances to reach the same height.
- If the second plane is made horizontal, then the sphere must travel forever trying to reach the required height.
This is the case when there is no unbalanced force acting on it.
From his experiments Galileo proposed that the body could travel indefinitely far as , contrary to the Aristotelian notion of the natural tendency of an object to remain at rest unless acted upon by an external force.
Therefore, Galileo can be credited with introducing the concept of inertia, later exploited by Newton.
However, in reality, frictional forces bring the sphere to rest after it travels over a finite distance.
After further study, Newton, in his first law of motion, stated that all objects resist a change in their natural state of motion.
This tendency of resisting any change in the natural state of motion is called “inertia”.
Newton’s Laws of Motion:
Newton studied the ideas of Galileo and gave the three laws of motion. These laws are popular as Newton’s laws of motion.
Newton’s First Law of Motion (Law of Inertia):
Any object remains in the state of rest or in the state of uniform motion along a straight line, until it is compelled to change its state by applying an external force.
Newton’s First Law of Motion in Everyday Life:
(a) A person standing in a bus falls backward when the bus starts suddenly.
This happens because the person and bus both are at rest while the bus is not moving, but as the bus starts moving, an external force is acted by the bus on the legs of the person. This external force moves legs along with the bus. But the rest of his body has the tendency to remain in rest known as inertia of rest. Because of this, the person falls backward; if he is not alert.
(b) A person standing in a moving bus falls forward if the driver applies brakes suddenly. This happens because when the bus is moving, the person standing in it is also in motion along with the bus. But when the driver applies brakes the speed of the bus decreases suddenly or the bus comes to a state of rest suddenly, in this condition the legs of the person which are in contact with the bus come to rest while the rest of his body have the tendency to remain in motion. Because this person falls forward if he is not alert.
(c) Before hanging the wet clothes over the laundry line, usually many jerks are given to the clothes to get them dried quickly. Because of jerks, droplets of water from the pores of the cloth fall on the ground and the reduced amount of water
in clothes dries them quickly. This happens because when suddenly clothes are made in motion by giving jerks, the water droplets in it have the tendency to remain in rest and they are separated from clothes and fall on the ground.
(d) When the pile of coins on the carrom-board is hit by a striker, the coin only at the bottom moves away leaving the rest of the pile of coins at the same place. This happens because when the pile is struck with a striker, the coin at
the bottom comes in motion while rest of the coin in the pile has the tendency to remain in the rest and they vertically falls the carrom-board
and remain at the same place.
Momentum
Momentum of an object at state of rest is zero :
Let an object with mass ‘m’ be at rest.
Since, object is at rest, its velocity, v = 0
We know that
Momentum, p is equal to the product of mass, m and velocity, v = 0
⇒ p = m × 0 = 0
Thus, the momentum of an object in the rest i.e., non-moving, is equal to zero.
Unit of momentum :
SI unit of mass = kg
SI unit of velocity = meter per second i.e., m/s
We know that Momentum (p) = m × v
⇒ p = kg × m/s
Or ⇒ p = kg m/s
Therefore, SI unit of momentum = kg m/s
Impulse and Impulsive Force
If a cricketer catches a ball he moves his hand back while catching the ball. He does this to reduce the impact, due to the force of the ball on his hand. An object in motion has momentum. Momentum is defined as the product of mass and velocity of an object.
The momentum of the object at the starting of the time interval is called the initial momentum and the momentum of the object at the end of the time interval is called the final momentum. The rate of change of momentum of an object is directly proportional to the applied force.
Newton’s second law quantifies the force on an object. The magnitude of force is given by the equation,
F = ma, where ‘m’ is the mass of the object and ‘a’ is its acceleration. The CGS unit of force is dyne and the SI unit is newton (N).
A large amount of force acting on an object for a short interval of time is called impulse or impulsive force. Numerically impulse is the product of force and time. Impulse of an object is equal to the change in momentum of the object.
Impulse and Impulsive Force
The momentum of an object is the product of its mass and velocity. The force acting on a body causes a change in its momentum. In fact, according to Newton’s second law of motion, the rate of change in the momentum of a body is equal to the net external force acting on it.
Another useful quantity that we come across is “impulse”. “Impulse” is the product of the net external force acting on a body and the time for which the force is acted.
If a force “F” acts on a body for “t” seconds, then Impulse I = Ft.
In fact, this is also equal to the change in the momentum of the body. It means that due to the application of force, if the momentum of a body changes from “P” to “P ‘ ”, then impulse,I = P ‘ – P.
For the same change in momentum, a small force can be made to act for a long period of time, or a large force can be made to act for a short period of time. A fielder in a cricket match uses the first method while catching the ball. He pulls his hand down along with the ball to decrease the impact of the ball on his hands.
In a cricket match, when a batsman hits a ball for a six, he applies a large force on the ball for a very short duration. Such large forces acting for a short time and producing a definite change in momentum are called “impulsive forces”.
Newton’s Second Law of Motion
Newton’s Second Law of Motion states that, the rate of change in momentum of an object is proportional to applied unbalanced force in the direction of force.
Mathematical expression:
State and derive newton’s second law of Motion
Statement: Newton’s second law of motion states that the rate of change of momentum of an object is Proportional to the applied unbalanced force in the direction of force.
Derivation of Newton’s second law of motion:
Suppose an object of mass, m is moving along a straight line with an initial velocity, u.
It is uniformly accelerated to velocity, v in time, t by the application of a constant force, F throughout the time t.
⇒Initial momentum of the object, p1 = mu
⇒Final momentum, p2 = mv
⇒Change in momentum = p2 – p1
⇒The change in momentum = mv – mu
⇒The change in momentum = m × (v – u)
⇒The rate of change of momentum = m(v -u)t
⇒ m (v -u)t
According to Newton’s Second Law of Motion,
Applied force α Rate of change in motion
⇒ F m (v -u)t
F=km (v -u)t = kma —————————- (i)
Here, k is a constant of proportionality and
(v -u)t is the rate of change of velocity, which equals acceleration, a.
The SI units of mass and acceleration are kg and m s-2 respectively.
The unit of force is so chosen that the value of the constant, K becomes one For this.
One unit of force is defined as the amount that produces acceleration
of 1 m s-2 in an object of 1 kg mass.
That is,
1 unit of force = k × (1 kg) × (1 m s-2).
Thus, the value of k becomes 1. From Eq. (iii)
F = ma ————————————-
The unit of force is kg m s-2 or newton, with the symbol N.
Newton’s Third Law of Motion
To every action there is an equal and opposite reaction.
Applications:
(i) Walking is enabled by 3rd law.
(ii) A boat moves back when we deboard it.
(iii) A gun recoils.
- Rowing of a boat.
Law of Conservation of Momentum
Law of conservation of momentum states that, if two or more bodies collide, the sum of the initial momentum is equal to the sum of the final momentum.
Or
Law of conservation of momentum states that the sum (total) of the individual momentums of the colliding bodies just before the collision is equal to the sum (total) of the individual momentums of the colliding bodies after the collision
Derivation of Law of Conservation of Momentum From Newton’s Third Law of Motion.
Answer:
For a system of bodies ( two or more bodies ), the total vector sum of momenta of all the bodies due to the mutual action and reaction remain unchanged as long as no external force is acted on the system.
Consider two bodies A and B of the masses m1, m2 moving with the initial velocities u1, u2 respectively.
For a system, let, these masses collide and their velocities after collision are v1, v2 respectively.
If ‘A’ applies a F on B for a time, t;
‘B’ applies a force –F on A for time t [according to Newton’s third law of motion].
Then,

Therefore, the sum of momentum before impact is equal to the sum of the momenta after the impact represents the law of conservation of momentum.
Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
GRAVITATION | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Content Study Tools Content … Key Terms Topic Terminology Term: Important Tables Topic Terminology Term: Assessments Test Your Learning readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Content
Study Tools
Content …
Hindi Version Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term:
WORK AND ENERGY | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Content : (Scroll down till end of the page) Study Tools Audio, Visual & Digital Content Content … Key Terms Topic Terminology Term Important Tables Table: . Assessments Test Your Learning readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Content : (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Content …
Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
SOUND | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Content : (Scroll down till end of the page) Study Tools Audio, Visual & Digital Content Content … Key Terms Topic Terminology Term Important Tables Table: . Assessments Test Your Learning readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Content : (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Content …
Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
STATISTICS | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Content Study Tools Content … Key Terms Topic Terminology Term: Important Tables Topic Terminology Term: Assessments Test Your Learning readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Content
Study Tools
Content …
Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term:
Assessments
Test Your Learning
SURFACE AREAS AND VOLUMES | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Plane figure The figures which we can be drawn on a flat surface or that lie on a plane are called Plane Figure. Example – Circle, Square, Rectangle etc. Solid figures The 3D shapes which occupy some space are called Solid Figures. Example – Cube, Cuboid,… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Plane figure
The figures which we can be drawn on a flat surface or that lie on a plane are called Plane Figure.
Example – Circle, Square, Rectangle etc.
Solid figures
The 3D shapes which occupy some space are called Solid Figures.
Example – Cube, Cuboid, Sphere etc. (Scroll down the till the end of the page)
Study Tools
Volume
Space occupied by any solid shape is the capacity or volume of that figure. The unit of volume is a cubic unit.
Surface Area
The area of all the faces of the solid shape is its total surface area. The unit of surface area is a square unit.
Lateral or Curved Surface Area
The surface area of the solid shape after leaving the top and bottom face of the figure is called the lateral surface of the shape. The unit of lateral surface area is a square unit.
Surface Area and Volume of a Cube
Cube is a solid shape having 6 equal square faces.
Lateral surface area of a cube 4s2 Total surface area of a cube 6s2 The volume of a cube s3 Diagonal √3 s, s = edge of the cube = side length of face of cube Surface Area and Volume of a Cube Example
What is the capacity of a cubical vessel having each side of 8 cm?
Solution
Given side = 8 cm So, Volume of the cubical vessel = l3 = (8)3 = 256 cm3.
Surface Area and volume of a Cuboid
Cuboid is a solid shape having 6 rectangular faces at a right angle.
Lateral surface area of a cuboid 2h(l + b) Total surface area of a cuboid 2(lb + bh + lh) Volume of a cuboid lbh Diagonal l = length, b = breadth, h = height Surface Area and volume of a Cuboid Example
What is the surface area of a cereal box whose length, breadth and height is 20 cm, 8 cm and 30 cm respectively?
Solution
Given, length = 20 cm, breadth = 8 cm, Height = 30 cm
Total surface area of the cereal box = 2(lb + bh + lh)
= 2(20 × 8 + 8 × 30 + 20 × 30)
= 2(160 + 240 + 600)
= 2(1000) = 2000 cm2.
Surface Area and Volume of a Right Circular Cylinder
If we fold a rectangular sheet with one side as its axis then it forms a cylinder. It is the curved surface of the cylinder. And if this curved surface is covered by two parallel circular bases then it forms a right circular cylinder.
Curved surface area of a Right circular cylinder 2πrh Total surface area of a Right circular cylinder 2πr2 + 2πrh = 2πr(r + h) The volume of a Right circular cylinder πr2h r = radius, h = height Surface Area and Volume of a Right Circular Cylinder Surface Area and Volume of a Hollow Right Circular Cylinder
If a right circular cylinder is hollow from inside then it has different curved surface and volume.
Curved surface area of a Right circular cylinder 2πh (R + r) Total surface area of a Right circular cylinder 2πh (R + r) + 2π(R2 – r2) R = outer radius, r = inner radius, h = height Surface Area and Volume of a Hollow Right Circular Cylinder Example
Find the Total surface area of a hollow cylinder whose length is 22 cm and the external radius is 7 cm with 1 cm thickness. (π = 22/7)
Solution
Given, h = 22 cm, R = 7 cm, r = 6 cm (thickness of the wall is 1 cm).
Total surface area of a hollow cylinder = 2πh(R + r) + 2π(R2 – r2)
= 2(π) (22) (7+6) + 2(π)(72 – 62)
= 572 π + 26 π = 598 π
= 1878.67 cm2
Surface Area and Volume of a Right Circular Cone
If we revolve a right-angled triangle about one of its sides by taking other as its axis then the solid shape formed is known as a Right Circular Cone.
Curved surface area of a Right Circular Cone πrl = πr[√(h2 + r2)] Total surface area of a Right Circular Cone πr2 + πrl = πr(r + l) The volume of Right Circular Cone (1/3) πr2h r = radius, h = height, l = slant height Surface Area and Volume of a Right Circular Cone Surface Area and Volume of a Sphere
A sphere is a solid shape which is completely round like a ball. It has the same curved and total surface area.
Curved or Lateral surface area of a Sphere 4πr2 Total surface area of a Sphere 4πr2 Volume of a Sphere (4/3) πr3 R = radius Surface Area and Volume of a Sphere Surface Area and Volume of a Hemisphere
If we cut the sphere in two parts then is said to be a hemisphere.
Curved or Lateral surface area of a Sphere 2πr2 Total surface area of a Sphere 3πr2 Volume of a Sphere (2/3) πr3 r = radius Surface Area and Volume of a Hemisphere Example
If we have a metal piece of cone shape with volume 523.33 cm3 and we mould it in a sphere then what will be the surface area of that sphere?
Solution
Given, volume of cone = 523.33 cm3
Volume of cone = Volume of Sphere
Volume of sphere = 100 π cm3
125 = r3
r = 5
Surface area of a sphere = 4πr2
= 314.28 cm2.
Hindi Version Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term:
Assessments
Test Your Learning
NUMBER SYSTEMS | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Introduction to Natural Numbers Non-negative counting numbers excluding zero are called Natural Numbers. N = 1, 2, 3, 4, 5, ………. Whole Numbers All natural numbers including zero are called Whole Numbers. W = 0, 1, 2, 3, 4, 5, ……………. (Scroll down till end of… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Introduction to Natural Numbers
Non-negative counting numbers excluding zero are called Natural Numbers.
N = 1, 2, 3, 4, 5, ……….
Whole Numbers
All natural numbers including zero are called Whole Numbers.
W = 0, 1, 2, 3, 4, 5, ……………. (Scroll down till end of the page)
Study Tools
Audio, Visual & Digital Content
Integers
All natural numbers, negative numbers and 0, together are called Integers.
Z = – 3, – 2, – 1, 0, 1, 2, 3, 4, …………..
Rational Numbers
The number ‘a’ is called Rational if it can be written in the form of r/s where ‘r’ and ‘s’ are integers and s ≠ 0,
Q = 2/3, 3/5, etc. all are rational numbers.
How to find a rational number between two given numbers?
To find the rational number between two given numbers ‘a’ and ‘b’.
Example:
Find 2 rational numbers between 4 and 5.
Solution:
To find the rational number between 4 and 5
To find another number we will follow the same process again.
Hence the two rational numbers between 4 and 5 are 9/2 and 17/4.
Remark: There could be unlimited rational numbers between any two rational numbers.
Irrational Numbers
The number ‘a’ which cannot be written in the form of p/q is called irrational, where p and q are integers and q ≠ 0 or you can say that the numbers which are not rational are called Irrational Numbers.
Example – √7, √11 etc.
Real Numbers
All numbers including both rational and irrational numbers are called Real Numbers.
R = – 2, – (2/3), 0, 3 and √2
Real Numbers and their Decimal Expansions
1. Rational Numbers
If the rational number is in the form of a/b then by dividing a by b we can get two situations.
a. If the remainder becomes zero
While dividing if we get zero as the remainder after some steps then the decimal expansion of such a number is called terminating.
Example:
7/8 = 0.875
b. If the remainder does not become zero
While dividing if the decimal expansion continues and not becomes zero then it is called non-terminating or repeating expansion.
Example:
1/3 = 0.3333….
Hence, the decimal expansion of rational numbers could be terminating or non-terminating recurring and vice-versa.
2. Irrational Numbers
If we do the decimal expansion of an irrational number then it would be non –terminating non-recurring and vice-versa. i. e. the remainder does not become zero and also not repeated.
Example:
π = 3.141592653589793238……
Representing Real Numbers on the Number Line
To represent the real numbers on the number line we use the process of successive magnification in which we visualise the numbers through a magnifying glass on the number line.
Example:
Step 1: The number lies between 4 and 5, so we divide it into 10 equal parts. Now for the first decimal place, we will mark the number between 4.2 and 4.3.
Step 2: Now we will divide it into 10 equal parts again. The second decimal place will be between 4.26 and 4.27.
Step 3: Now we will again divide it into 10 equal parts. The third decimal place will be between 4.262 and 4.263.
Step 4: By doing the same process again we will mark the point at 4.2626.
Operations on Real Numbers
1. The sum, difference, product and quotient of two rational numbers will be rational.
Example:
2. If we add or subtract a rational number with an irrational number then the outcome will be irrational.
Example:
If 5 is a rational number and √7 is an irrational number then 5 + √7 and 5 – √7 are irrational numbers.
3. If we multiply or divide a non-zero rational number with an irrational number then also the outcome will be irrational.
Example:
If 7 is a rational number and √5 is an irrational number then 7√7 and 7/√5 are irrational numbers.
4. The sum, difference, product and quotient of two irrational numbers could be rational or irrational.
Example:
Finding Roots of a Positive Real Number ‘x’ geometrically and mark it on the Number Line
To find √x geometrically
1. First of all, mark the distance x unit from point A on the line so that AB = x unit.
2. From B mark a point C with the distance of 1 unit, so that BC = 1 unit.
3. Take the midpoint of AC and mark it as O. Then take OC as the radius and draw a semicircle.
4. From the point B draw a perpendicular BD which intersects the semicircle at point D.
The length of BD = √x.
To mark the position of √x on the number line, we will take AC as the number line, with B as zero. So C is point 1 on the number line.
Now we will take B as the centre and BD as the radius, and draw the arc on the number line at point E.
Now E is √x on the number line.
Identities Related to Square Roots
If p and q are two positive real numbers
Examples:
1. Simplify
We will use the identity
2. Simplify
We will use the identity
Rationalising the Denominator
Rationalize the denominator means to convert the denominator containing square root term into a rational number by finding the equivalent fraction of the given fraction.
For which we can use the identities of the real numbers.
Example:
Rationalise the denominator of 7/(7- √3).
Solution:
We will use the identity
here.
Laws of Exponents for Real Numbers
If we have a and b as the base and m and n as the exponents, then
1. am × an =am+n
2. (am)n = amn
4. am bm = (ab)m
5. a0 = 1
6. a1 = a
7. 1/an = a-n
- Let a > 0 be a real number and n a positive integer.
- Let a > 0 be a real number. Let m and n be integers such that m and n have no common factors other than 1, and n > 0. Then,
Example:
Simplify the expression (2x3y4) (3xy5)2.
Solution:
Here we will use the law of exponents
am × an =am+n and (am)n = amn
(2x3y4)(3xy5)2
(2x3y4)(3 2 x 2 y10)
18. x3. x2. y4. y10
18. x3+2. y4+10
18x5y14
Hindi Version Key Terms
Topic Terminology
Term
Important Tables
Table:
.
Chemical Reactions and Equations | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Any process that involves the rearrangement of structure of the substance or conversion of reactants into products is defined as Chemical Reaction. For a Chemical Reaction to occur, the change can be observed in the form of – Content Study Tools Audio, Visual & Digital Content… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Any process that involves the rearrangement of structure of the substance or conversion of reactants into products is defined as Chemical Reaction.
For a Chemical Reaction to occur, the change can be observed in the form of –
- Change in State: Melting of ice into water.
- Change in Colour: Iron rusting which has colour change from silver to reddish brown. (Scroll down till the end)
Content
Study Tools
Audio, Visual & Digital Content
Chemical Reactions and Equations | Study Tools
Chemical Reactions and Equations
Any process that involves the rearrangement of structure of the substance or conversion of reactants into products is defined as Chemical Reaction.
For a Chemical Reaction to occur, the change can be observed in the form of –
- Change in State: Melting of ice into water.
- Change in Colour: Iron rusting which has colour change from silver to reddish brown.
- Change in Temperature: There are two types of reaction i.e Exothermic and Endothermic Reaction.
Exothermic Reactions: Those reactions in which energy is released in the form of heat are called Exothermic Reactions.
Examples –
(1) All combustion reactions e.g.
CH4+ 2O2 —> CO2 + 2H2O + Heat
(2) Thermite reactions e.g.
2A1 + Fe2O3 —> 2Fe + Al2O3 + Heat
Combinations are generally exothermic in nature. The decomposition of organic matters into compost is an example of exothermic reaction.
Endothermic Reactions: Those reactions in which energy is absorbed are called Endothermic Reactions.
Examples –
also, the reaction of photosynthesis –
- Evolution of any gas: When Zinc reacts with sulphuric acid it gives hydrogen gas.
Zn + H2 SO4 → ZnSO4 + H2
Formation of Precipitate: When a soluble carbonate reacts with Barium, Barium Carbonate precipitate can be observed.
Change in State
Some chemical reactions are characterised by a change in state.
- When wax is burned (in the form of wax candle,) then water and carbon dioxide are formed.
- Now, wax is a liquid whereas carbon dioxide is a gas. This means that during the combustion reaction of wax, the physical state changes from solid to liquid and gas.
Physical Change
- In this change identity of the substance remains same.
- For Example, Melting, Boiling etc.
Chemical Change
- The identity of the substances change
- Reactants are converted into substance due to formation or broken down of older bonds
Chemical Equation
The symbolic representation of chemical reaction using symbols and formulae is known as Chemical Equation. For this, reactants are written on the left hand side whereas products are written on the right.
Balanced Chemical Equation
A balanced chemical equation is the one where the number of atoms involved in reactants side is equal to number of atoms on product side.
Eq.1. Example of Balanced Chemical Equation
Steps to form Balanced Equation
To show how to balance the equation, the following equation is used-
Fe + H2O → Fe3O4 + H2
Step 1: First of all, draw the boxes around each formula as shown below-
Step 2: Find out the number of atoms of each element. For Example, on reactant side, 1 for Fe, 2 H, and 1 O and on product side we have, 3 for Fe, 4 for O and 2 for H.
Step 3: Start to balance the equation with the compound having maximum number of atoms. While balancing does not alter the formula of the compound.
Step 4: One by one balance each element on reactant and product side.
Step 5: After balancing number of atoms on both the side of the equation, finally check the correctness of the balanced equation.
Step 6: then write the symbols of the physical state of reactants and products as shown below-
3Fe(s) + 4H2O (g) → Fe3O4 (s) + 4H2 (g)
This above equation represents the balanced equation.
Balancing a Redox Reaction
The basic ionic form of the equation is-
Fe2+ + Cr2O72- → Fe3+ + Cr3+
Oxidation half reaction is-
Reduction half reaction is-
Use the reduction half method to balance the equation. Balance the atoms in each half of the reaction except H and O atoms.
Cr2O72- (aq) → 2 Cr3+(aq)
Add water molecules as the reaction is taking place in acidic solution. This is to balance the O atoms and hydrogen ions.
Cr2O72- (aq) + 14 H+(aq) → 2 Cr3+(aq) + 7H2O (I)
Then balance the charges in both half reactions.
Fe2+(aq) → Fe3+(aq) + e–
Cr2O72- (aq) + 14 H+ + 6e– → 2 Cr3+ + 7H2O
6 Fe2+(aq) → 6 Fe3+(aq) + 6e–
Two half of the equations are added to get the overall reaction
6Fe2+(aq) + Cr2O72-(aq) + 14H+(aq) → 6Fe3+(aq) + 2Cr3+(aq) + 7H2O (I)
Types of Chemical Reaction
- Combination Reaction is reaction when single product is formed from the combination of two or more reactants. For Example-
Eq.2. Example of Combination Reaction
Reactions can be exothermic as well as endothermic. Exothermic reaction release heats and raises the temperature of the surroundings. For Example, Respiration is an example of exothermic reaction.
Eq.3. Example of Exothermic Reaction
Endothermic reaction involved the absorption of the heat and thus it cools the surrounding. The decomposition of dead organic material is an endothermic reaction.
- Decomposition Reaction is type of reaction which involves breakdown of single reactant into simpler products. Decomposition of silver chloride into silver and chlorine in presence of sunlight is an example of decomposition reaction.
Eq.4. Example of Decomposition Reaction
- Displacement Reaction is a reaction in which more reactive element will displaces the less reactive element.
Eq. 5. Example of Displacement Reaction
- Double Displacement Reaction is a type of reaction in which cations and anions in the reactants switch the places to form new products.
Eq. 6. Example of Double Displacement Reaction
- Redox Reaction is also known as Oxidation-reduction Reaction. In this type of reaction transfer of electrons occurs between the two species. Oxidation is defined as addition of oxygen or removal of hydrogen. Reduction is defined as removal of oxygen or addition of hydrogen. Oxidizing agent is the one which gains the electrons and is reduced in a chemical reaction. Reducing agent is oxidized in a chemical reaction and it loses the electrons. Fluorine is the strongest oxidizing agent. Formic acid is a reducing agent
Eq.7. Example of Redox Reaction
Corrosion
Metals are prone to corrosion. It is a slow conversion of metals into some undesirable compounds. This occur may be due to reaction with oxygen, gases, acids etc. When irons reacts with atmospheric oxygen and moisture, a red layer is formed on the surface of the iron, this process is known as Rusting.
Eq. 8. Equation for Iron Rusting
Rancidity
When food containing fats and oils are exposed to the atmosphere, the oxidation of fat and oil occurs, this is known as Rancidity.
Methods to Prevent Rancidity
- Store cooking oils from direct sunlight.
- Food should be placed at low temperature.
- By adding antioxidants food can be protected from rancidity.
- Chemical Reactions and Equations
- Minimize the use of salts in fried foods.
Hindi Version Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term:
Acids, Bases and Salts | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage The taste of the food is due to the presence of acids and bases in them. Acids Content Study Tools Audio, Visual & Digital Content ACIDS, BASES AND SALTS | FULL NOTES The taste of the food is due to the presence of acids and bases… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
The taste of the food is due to the presence of acids and bases in them.
Acids
- Acids are defined as the substances which produce hydrogen ions or Hydronium ions (H3O+) in water. For Example, Sulphuric Acid, Hydrochloric, Nitric Acid, Acetic Acid etc.
- They taste sour.
- Acids turn blue litmus to red. This is used as a confirmation test for the presence of acid.
- When acids react with metals, gases are evolved. (Scroll down till the end…)
Content
Study Tools
Audio, Visual & Digital Content
ACIDS, BASES AND SALTS | FULL NOTES
The taste of the food is due to the presence of acids and bases in them.
Acids
- Acids are defined as the substances which produce hydrogen ions or Hydronium ions (H3O+) in water. For Example, Sulphuric Acid, Hydrochloric, Nitric Acid, Acetic Acid etc.
- They taste sour.
- Acids turn blue litmus to red. This is used as a confirmation test for the presence of acid.
- When acids react with metals, gases are evolved.
Reactions with Acids
1. Reaction of Acid with Metal
Acid + Metal → Salt + Hydrogen gas
Mg + H2SO4 → H2 + MgSO4
2. Reaction of Acid with Carbonates
Na2CO3 (s) + 2 HCl (aq) → 2NaCl (aq) + H2O(l) + CO2(g)
3. Reaction of Acid with Bicarbonates
NaHCO3 (s) + HCl (aq) → NaCl(aq) + H2O (l) + CO2 (g)
Similarity between Acids and Bases
- Both acids and bases react with water. They produce ions in water
- Both acids and bases act as electrolytes, so are good conductors of electricity.
- Both of them change the colour of the litmus paper.
Classification of Acids
Acids are classified as Organic Acids and Mineral Acids. Acids which are derived from plants and animals, they are known as Organic Acids.
Example, Citric Acid from fruit.
Mineral acids are inorganic acids such as Sulphuric Acid. They are dangerous to be used, so need more precautions.
Acids are also classified as Strong Acids or Weak Acids. Strong acid is an acid that completely dissociates into ions in aqueous solutions. For Example, Sulphuric Acid, Hydrochloric Acid.
Weak acid is the one which does not dissociate completely into ions in aqueous solutions. For Example, Acetic Acid.
Acids can also be as Dilute Acid and Concentrated Acids. The one which has low concentration of acids in aqueous solution, they are known as Dilute Acids whereas the one which has high concentration of acids in aqueous solution, are known as Concentrated Acids.
It is advisable to add acid to water and not vice versa because a large amount of heat is released if water is added to acid. This released heat is large enough to cause harm.
Acids can also be classified based on the number of hydrogen ions. Monoprotic acid is the one which gives one mole of hydrogen ions per mole of acid, such as HCl. Diprotic Acid is the one which produces two mol of hydrogen ions per mole of acid. For Example, H2SO4.
Bases
- Bases are the one which produces hydroxide ions in aqueous solutions. Bases which are water soluble are known as Alkalis.
- They turn red litmus to blue.
- They have a bitter taste.
- They also produced carbon-dioxide when reacted with carbonates.
- They also evolved hydrogen gas when bases react with metals.
Reactions of Bases
1. Reaction with Metals
Base reacts with metals and produces hydrogen gas.
2NaOH + Zn → Na2 → Na2ZnO2 + H2
2. Reaction with Acids
Base reacts with acids to form salts. For Example,
KOH + HCl → KCl + H2O
3. Reaction with Non-metallic Oxides
Base reacts with non-metallic oxides to form salt and water.
2NaOH + CO2 → CO2 → Na2CO3 + H2O
Classification of Bases
Bases are classified as Strong Base and Weak Base. Strong base is the one which dissociates completely into its ions in aqueous solution. For Example, NaOH.
Weak base is the one which does not dissociate completely into its ions in aqueous solutions. For Example, Ammonium Hydroxide, NH4OH
Bases are also classified as Dilute Base and Concentrated Base. The solution which has low concentration of base in aqueous solution is defined as Dilute Base whereas the one which has high concentration of base in aqueous solution is known as Concentrated Base.
Strength of Acid or Base Solutions
The dissociation constant of weak acid or weak base can be represented as-
Suppose HA is weak acid, then dissociation constant is represented as-
Strength of an acid or base can be determined using a pH scale. It is a scale to measure the hydrogen ion concentration in a solution. The p stands for ‘potenz’, it is a German word which means power.
- If pH is equal to 7, means the solution is neutral.
- If pH is greater then 7 means alkaline solution.
- If pH is less then 7 means the solution is acidic.
Fig.1. pH scale
Importance of pH
- Human body works at a pH of about 7.4.
- Stomach has a pH of about 2 due to the presence of hydrochloric acid in it. It is needed for the activation of pepsin protein required for protein digestion.
- When we eat food containing sugar, then the bacteria present in our mouth break down the sugar to form acids. This acid lowers the pH in the mouth. Tooth decay starts when the pH of acid formed in the mouth falls below 5.5. This is because then the acid becomes strong enough to attack the enamel of our teeth and corrode it. This sets in tooth decay. The best way to prevent tooth decay is to clean the mouth thoroughly after eating food.
- Many animals and plants protect themselves from enemies by injecting painful and irritating acids and bases into their skin.
- When a honey bee stings a person, it injects an acidic liquid into the skin. Rubbing with a mild base like baking soda solution on the stung area of the skin gives relief.
- When a wasp stings, it injects an alkaline liquid into the skin. Then rubbing with a mild acid like vinegar on the stung area of the skin gives relief.
- Soil pH and plant growth: Most of the plants grow best when the pH of the soil is close to 7. If the soil is too acidic or basic, the plants grow badly or do not grow at all. The soil pH is also affected by the use of chemical fertilisers in the field. Chemicals can be added to soil to adjust its pH and make it suitable for growing plants. If the soil is too acidic then it is treated with materials like quicklime or slaked lime. If the soil is too alkaline then alkalinity can be reduced by adding decaying organic matter.
Salts
When acid and base neutralise, salts are formed. Strong acid and strong base combine to form neutral salt.
NaOH + HCl → NaCl + H2O
Eq.1. Formation of Neutral Salt
Strong acid and weak base combine to form acidic salt. For Example, Hydrochloric Acid and ammonium hydroxide combine to form ammonium chloride. Other examples, sodium hydrogen carbonate, sodium hydrogen sulphate etc.
HCl + NH4OH → NH4Cl + H2O
Eq.2. Formation of Acidic Salt
Similarly, weak acid and strong base combine to form basic salt. For Example, Acetic Acid and sodium hydroxide combine to form sodium acetate. Other examples are calcium carbonate, potassium cyanide etc.
CH3COOH + NaOH → CH3COONa + H2O
Eq.3. Formation of Basic Salt
The most common salt is table salt or sodium chloride (NaCl).
Indicators
They are the substances that indicate acidic or basic nature of the solution using colour change. For Example, litmus solution, methyl orange, phenolphthalein, methyl red etc. Acids convert blue litmus paper red in colour. Bases turn red litmus blue. Phenolphthalein remains colourless in presence of acids but turns pink in presence of bases.
Some Important Chemical Compounds and their uses
Salt Preparation Uses Common Salt (Sodium Chloride) (NaCl) 1. NaOH + HCl → NaCl + H2O2. From sea water by evaporation3. From underground deposit{Large crystals of common salt found in underground deposits which are brown due to presence of impurities in it. It is mined from underground deposits like coal.} 1. Raw material for making large numbers of useful chemicals in industry. Eg: NaOH (caustic soda), Na2CO3 (washing soda), NaHCO3 (baking soda).2. Preservative in pickle and curing meat and fish.3. To melt ice and clear roads in winters in cold countries.4. Used in the manufacturing of soap. Caustic Soda (NaOH)(Sodium Hydroxide) Passing electricity through concentrated solution of NaCl (called ‘brine’) 2NaCl (Brine) + 2H2O 2NaOH (Caustic Soda) + Cl2 + H2At anode (+ve electrode): Cl2 is producedAt cathode (-ve electrode): H2 is producedIt is called chloro-alkali process because products formed are chlorine (Chloro) and NaOH (alkali).
Uses of H21. Hydrogenation of oil to get vegetable ghee (margarine)2. To make ammonia for fertilisers3. In fuel for rockets.Uses of Cl21. In water treatment2. To clean water in swimming pools3. To make plastic, e.g. PVC4. To make CFCs, chloroform, dyes etc.Uses of NaOH1. Used in making soap and detergent.2. Used in manufacturing of paper3. De-greasing metals4. Refining oil5. Making dyes and bleachesUses of HCl1. Cleaning steel2. Preparation of chloride, e.g. NH4Cl3. In making medicines and cosmetics4. In making plastics, PVC etc. Baking Soda (NaHCO3)(Sodium Hydrogen Carbonate) NaCl + NH3 + H2O + CO2 → NaHCO3 + NH4ClPropertiesAction of Heat: 1. Used as antacid in medicine to remove acidity of the stomach2. Used in making baking powder (Basic soda + tartaric acid)NaHCO3 + H⊕ (from mild acid) → Na⊕ (sodium salt of acid) + CO2 + H2OThe CO2 produced during the process gets trapped in wet dough and bubbles out slowly to make the cake ‘rise’ so that it becomes soft and spongy.Tartaric acid neutralises it, and so it has a pleasant taste.3. Used in soda-acid fire extinguisher Washing Soda (Na2CO3.10H2O)(Sodium Carbonate) Na2CO3 + 10 H2O → Na2CO3.10H2OPreparation of Na2CO3{NaCl + NH3 + H2O + CO2 NaHCO3 + NH4ClNaHCO3 → Na2CO3 + CO2 + H2O} 1. Used in glass, soap and paper industries2. Used in manufacturing of sodium compounds such as Borax3. Cleaning agent for domestic purpose4. Remove permanent hardness of water Bleaching Powder (CaOCl2)Calcium Oxychloride Ca(OH)2 + Cl2 → CaOCl2 + H2OSlaked Lime Calcium OxychloridePropertiesCaOCl2 + H2SO4 → CaSO4 + Cl2 + H2OThe Cl2 produced by action of dilute acid acts as a bleaching agent. 1. For bleaching cotton and linen in textile industry, for bleaching wood pulp in paper factories, for bleaching washed clothes in laundry2. Oxidising agent in chemical industries3. Disinfecting drinking water Plaster of Paris (P.O.P) (CaSO4.1/2 H2O)(Calcium Sulphate Hemihydrate) CaSO4.H2O (Plaster of Paris) +3/2 H2O* Heating of gypsum should not be done above 100oC as above that temperature, water of crystallisation will be eliminated and anhydrous CaSO4 will be obtained. This anhydrous CaSO4 is known as Dead Burnt Plaster.* CaSO4.1/2 H2O means that two molecules of CaSO4 share one molecule of water.PropertiesHas the remarkable property of setting into a hard mass on wetting with water, as gypsum is formed.CaSO4.1/2 H2O (P.O.P) + 1/2 H2O → CaSO4.2H2O (Gypsum set as hard mass)Hence, P.O.P should be stored in moisture-proof containers as moisture can cause slow setting of P.O.P by hydrating it.
1. Used in hospital for setting fractured bones in the right position to ensure correct healing.2. Making toys, decorative materials, cheap ornaments, and casts of statues.3. Used as fire-proofing material4. Used in chemistry labs for setting air gaps in apparatus.5. Making smooth surfaces, such as For making ornamental designs on ceilings of houses and other buildings Hindi Version Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term:
Metals and Non-metals | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Elements are classified as metals and non-metals based on different properties. The properties of metals and non-metals are given in the form of table below- Metals Non-metals Metals are lustrous, that is, they have a property to shine. They are not lustrous, that is, they do… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Elements are classified as metals and non-metals based on different properties. The properties of metals and non-metals are given in the form of table below-
Metals Non-metals Metals are lustrous, that is, they have a property to shine. They are not lustrous, that is, they do not have shining surface. except, graphite and iodine All metals exist as solids except mercury which is liquid at room temperature. They are generally soft, except diamond. They can be drawn into wires, this is known as Ductility. They are non-ductile. Metals can be converted into sheets, this is known as Malleability, except mercury They are non-malleable They are good conductors of electricity and heat. Except Lead and mercury. They are poor conductors of electricity and heat. Exception-graphite is good conductor of electricity They have high density and high melting point. Exception-sodium and potassium have low melting points They have low density compared to metals and low melting point except Diamond which has high melting point Study Tools
Audio, Visual & Digital Content
Elements are classified as metals and non-metals based on different properties. The properties of metals and non-metals are given in the form of table below-
Metals Non-metals Metals are lustrous, that is, they have a property to shine. They are not lustrous, that is, they do not have shining surface. except, graphite and iodine All metals exist as solids except mercury which is liquid at room temperature. They are generally soft, except diamond. They can be drawn into wires, this is known as Ductility. They are non-ductile. Metals can be converted into sheets, this is known as Malleability, except mercury They are non-malleable They are good conductors of electricity and heat. Except Lead and mercury. They are poor conductors of electricity and heat. Exception-graphite is good conductor of electricity They have high density and high melting point. Exception-sodium and potassium have low melting points They have low density compared to metals and low melting point except Diamond which has high melting point Chemical Properties of Metals
- Metals react with air or oxygen to form metal oxide.
For Example, Copper reacts with oxygen to form copper oxide.
Metal + O2 → Metal oxide
2Cu + O2 → 2CuO
4Al + 3O2 → 2Al2O3
- Oxides of metals can react with both acids and bases to produce salt and water. Such oxides are known as Amphoteric Oxides.
Al2O3 + 6HCl → 2AlCl3 + H2O
- Metals also reacts with water to form metal oxide. Metal oxide in turn can react with water to form metal hydroxide. For Example
2Na + 2H2O → 2NaOH + 1H2
2Al + 3H2O → Al2O3 + 3H2
- Metals also reacts with dilute acids to form salt and hydrogen. For example, magnesium reacts with dilute hydrochloric acid to form magnesium chloride and hydrogen.
Metal + Acid → Metal Salt + Hydrogen
Mg + 2HCl → MgCl2 + H2
Chemical Properties of Nonmetals
- Non-metals react with oxygen to form non-metal oxide.
Non-metal + Oxygen → Non-metal oxide
C + O2 → CO2
- Non-metals do not react with water and acids to evolve hydrogen gas.
- Non-metals can react with salt solution; more reactive elements will displace the less reactive non-metal.
2 NaBr (aq) + Cl2(aq) → 2NaCl (aq) + Br2 (aq)
- Non-metals can also react with hydrogen to form hydrides.
H2(g) + S(l) → H2S(g)
Reactivity Series
The series in which metals are arranged in the decreasing order of reactivity, it is known as Reactivity Series.
Fig.1. Reactivity Series
Ionic Compounds
Compounds formed due to the transfer of electrons from a metal to a non-metal are known as Ionic Compounds.
Covalent Bond
Bond formed by sharing of electrons between the two atoms. They share their valence electrons to gain stability.
Properties of Ionic Compounds
- They are generally hard and solid.
- They have a high melting and boiling point.
- They are soluble in water but insoluble in inorganic solvents such as ether etc.
- They are conductors of electricity in molten and solution state.
Occurrence of Metals
Elements or compounds which occur naturally in earth crust are known as Minerals. Minerals from which pure metals can be extracted are known as Mineral Ores.
Extraction of pure metals from its ores/steps for extraction of metals from its ore
- The first step is enrichment of the ore.
- Second step includes extraction of metals
- Third steps involve refining of metal
Gangue – Ores contain different impurities in it such as sand, soil etc. These impurities are known as Gangue.
Extracting Metals which are low in activity series
Metals which are low in activity series are unreactive. The oxides of such metals can be reduced to metals by heating alone. For Example, Cinnabar (HgS)
Extracting Metals in the middle of the Activity Series
These metals are moderately reactive. They exists as sulphides or carbonates in nature. Before reduction, metal sulphides and carbonates must be converted into metal oxides. Sulphide ores are converted into oxides by heating strongly in presence of excess air, this is known as Roasting. Carbonate ores are converted into oxides by heating in limited air. This is known as Calcination.
Roasting
Calcination
Reduction-metal oxides can be reduced to metals using reducing agent such as such as Carbon.
Extracting metals towards the top of the activity series
The metals are highly reactive. They cannot be obtained by heating. For Example, Sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides.
At cathode Na+ + e– → Na
At anode 2Cl– → Cl2 + 2e–
Refining of Metals
Refining of impure metal is done using electrolytic refining. Impure copper is used as anode and strip of pure copper is used as Cathode. Acidified copper sulphate is used as electrolyte. When electric current is passed through this, impure metal from the anode gets deposited in the electrolyte solution, whereas pure metal from the electrolyte is deposited at cathode.
Deposition of insoluble residue formed from the dissolution of anode during commercial electrolysis.
Electrolytic refining
Corrosion
Metals when exposed to moist air for a long period of time, they become corroded. This is known as Corrosion. For Example, Silver reacts with moist air and becomes black in colour due to silver sulphide coating.
Iron + oxygen → Iron (III) oxide
Fe + O 2 → Fe2O3
Prevention of Corrosion
- Rusting of iron can be prevented by oiling, galvanising, painting, greasing etc.
- To protect steel and iron from rusting, a thin layer of zinc is coated on them, this is known as Galvanization.
Alloy
Mixture of two or more metals or metal and non-metal is known as Alloy. For Example,
- Brass is an alloy of copper and zinc.
- Bronze in an alloy of copper and tin.
- Solder is an alloy of lead and tin.
- Amalgam is one metal that is mercury.
Hindi Version Carbon and its Compounds | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Two or more elements combine to form compounds. There are two types of compounds- Organic Compound and Inorganic Compounds. Organic compounds are the one which are made up of carbon and hydrogen. (Scroll down till the end…) Study Tools Audio, Visual & Digital Content Revision Notes… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Two or more elements combine to form compounds. There are two types of compounds- Organic Compound and Inorganic Compounds. Organic compounds are the one which are made up of carbon and hydrogen. (Scroll down till the end…)
Study Tools
Audio, Visual & Digital Content
Revision Notes on Carbon and its Compounds
Two or more elements combine to form compounds. There are two types of compounds- Organic Compound and Inorganic Compounds. Organic compounds are the one which are made up of carbon and hydrogen.
Covalent Bond
The bond formed by sharing a pair of electrons between two atoms is known as Covalent Bond. Carbon forms a covalent bond. Carbon exists in two forms- as free state and as combined state. Free form of carbon is found in graphite, diamond and fullerene. In a combined state, carbon exists as Carbon-dioxide, Glucose, Sugar etc.
Allotropes of Carbon
Different forms of an element that has same chemical properties but different physical properties are known as Allotropes. There are three allotropes of carbon- diamond, graphite and fullerene.
Diamond
Diamond exits as three-dimensional network with strong carbon-carbon covalent bonds. Diamond is hard in nature with high melting point. It shines in presence of light and it is a bad conductor of electricity. The most common use of diamond is in making jewellery. It is also used in cutting and drilling tools.
Graphite
Graphite is made from weak van der wall forces. Each carbon atom is bonded with other three carbon atoms in order to form hexagonal rings. It serves as good conductor of heat and electricity. It is used as dry lubricant for machine parts as well as it is used in lead pencils.
Fullerene
It is a hollow cage which exits in the form of sphere. Its structure is similar to fullerene. But along with hexagonal rings, sometimes pentagonal or heptagonal rings are also present.
Fig.1 Structure of fullerene
Two Important Properties of Carbon
Catenation and tetravalency are the two important properties of carbon.
Catenation is a property of carbon by which carbon atoms can link one another via covalent bond and can form long chains, closed rings or branched chains etc.
Multiple Bonds:
Carbon atoms can be linked by single, double or triple bonds.
Carbon has a valency of 4 due to which it is known to have tetravalency. Due to this one carbon atom can bond with other 4 carbon atoms, with other atoms also such as Oxygen, Nitrogen etc.
Hydrocarbons
Compounds which are made up of carbon and hydrogen are known as Hydrocarbons.
There are two types of hydrocarbons found – Saturated Hydrocarbons and Unsaturated Hydrocarbons.
Saturated Hydrocarbons
Saturated Hydrocarbons consist of single bonds between the carbon atoms.
For Example, Alkanes. Alkanes are saturated hydrocarbons.
General formula of saturated hydrocarbons
CnH2n+2.
Unsaturated Hydrocarbons are the one with double or triple bonds between the carbon atoms.
For Example, Alkenes and Alkynes. Alkenes are represented as CnH2n whereas alkynes are represented as CnH2n-2. Some saturated hydrocarbons and unsaturated hydrocarbons are represented as –
Fig.2. Saturated hydrocarbons
Fig. 3. Unsaturated hydrocarbons
Structure of hydrocarbons can be represented in the form of electron dot structure as well as open structures as shown below-
Fig.4. Electron dot structure and open structure of ethane
Fig.5. Electron dot structure and open structure of ethyne
Carbons Compounds based on the basis of structure
Carbon Compounds can be classified as straight chain compounds, branched chain compounds and cyclic compounds.They are represented as –
Fig.6. Straight chain carbon compound
Fig.7. Branched chain compounds
Fig.8. Cyclic carbon compounds
Functional Groups
One of the hydrogen atoms in hydrocarbon can be replaced by other atoms according to their valencies. The atoms which decides the properties of the carbon atoms, are known as Functional Groups. For Example, Cl, Br, -OH, Aldehyde, Ketone, Carboxylic Acid etc.
Homologous Series
Series of compounds in which same functional group substitutes for the hydrogen atom in a chain of carbon.
Fig.9. Homologous series
Nomenclature of Carbon Compounds
- First of all, identify the number of carbon atoms in compounds. And in it identify the longest chain
- Then functional group can be indicated by suffix or prefix.
- Cyclic hydrocarbon is designated by prefix cyclo.
- If there are two or more different substituents they are listed in alphabetical order
- If the same substituent occurs more than once, the location of each point on which the substituent occurs is given
Fig.10. Different functional groups
Chemical Properties of Carbon Compounds
Combustion
Carbon along with its compound is used as a fuel as it burns in presence of oxygen to release energy. Saturated hydrocarbons produce blue and non-sooty flame whereas unsaturated hydrocarbons produce yellow sooty flame.
CH4 + 2O2 → CO2 + 2H2O
Oxidation
Alcohol can be oxidized to aldehydes whereas aldehydes in turn can be oxidized to carboxylic acid. Oxidizing agent such as potassium permanganate can be used for oxidation.
Addition Reaction
Hydrogenation of vegetable oil is an example of addition reaction. Addition of hydrogen in presence of catalyst such as nickel or palladium. This converts oil into ghee.
Substitution Reaction
When one atom in hydrocarbon is replaced by chlorine, bromine, etc. this is known as Substitution Reaction.
Important Carbon Compounds: Ethanol and Ethanoic Acid
Ethanol is a volatile liquid with low melting point. It reacts with sodium to form sodium ethoxide.
This above reaction is used to test the presence of ethanol by the evolution of hydrogen gas.
Dehydration of ethanol in presence of hot sulphuric acid forms alkene.
Ethanoic acid is a colourless liquid. When pure ethanoic acid freeze like ice, it is known as Glacial Acetic Acid. It is formed at a temperature of about 16.6 degree centigrade
Ethanoic Acid/Acetic acid when reacts with ethanol it forms an ester. Ester can be identified by its sweet smell.
Reaction of ester with strong base is used to form soap. This is known as Saponification. Acetic acid also reacts with strong base to form sodium acetate and water.
NaOH + CH3COOH + CH3COONa + H2O
Soaps and Detergents
Sodium or potassium salt of carboxylic acid is known as Soap. They work most effectively in soap water. Detergents are sulphonate or ammonium salt of long chain of carboxylic acid. They can work effectively on soft as well as hard water.
Cleansing Action of Soaps and Detergents
Cleansing action of soaps and detergents is due to ability to minimize the surface tension of water, to emulsify oil or grease and to hold them in a suspension of water. When soap dissolves in water, it forms soap anions and soap cations. The hydrophobic part of soaps and detergents are soluble in grease and hydrophilic part is soluble in water.
Soap and Micelle Formation
When dirt and grease are mixed with soap water, soap molecules arrange them in tiny clusters known as Micelle. The hydrophilic part sticks to the water and form outer surface of the micelle and hydrophobic part binds to oil and grease.
Hindi Version Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term:
Life Processes | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Biology is the study of living things. All living things are called organisms, both plants and animals are living organisms. But how we decide whether something is living or non-living depends on 7 lifeprocesses. If something is living it will carryout the 7 life processes below.… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Biology is the study of living things. All living things are called organisms, both plants and animals are living organisms. But how we decide whether something is living or non-living depends on 7 lifeprocesses. If something is living it will carryout the 7 life processes below. (Scroll down till end ..)
Content
Study Tools
Audio, Visual & Digital Content
What are life processes?
Biology is the study of living things. All living things are called organisms, both plants and animals are living organisms. But how we decide whether something is living or non-living depends on 7 lifeprocesses. If something is living it will carryout the 7 life processes below.
1.Movement Both animals and plants have the ability to move. Plants are rooted and move slowly as theygrow. Their roots move down into the soil and their stems moveup towards thelight. Animals onthe other handmove quickly andcan move their entire bodies. They canmove in search of food, shelter or to avoiddanger.
2. Respiration Respiration is theprocess of extracting energy out ofthe food weeat. All living things respire because they need energy to grow, to replace worn out parts and to move. Respiration takes placein the mitochondria of the cell.
3. Sensitivity All living organisms are sensitive; this means that they have anawareness of changes in their environment. Animals respond quickly to stimuli such as heat, light, sound, touch and chemicals which have taste and smell. On the other hand, plants generally appear less sensitive and their response is slower.
4. Growth All living organisms grow. Plants continue growing throughout their lives. Animals stop growing once they reach adulthood. Even when growth stops, materials within an animal’s body arestill being replaced from its food.
5. Excretion All living things make waste products these can beuseless or harmful to it and therefore need to be got rid of. Excretion is the process of getting rid of metabolic waste. Plants store waste substances in their leaves, the waste is removed when their leaves fall off. Animals breathe out waste carbon dioxide, otherwaste substances leavethe body in urineand sweat. Note: Getting rid offaeces or undigested food is notexcretion but egestion.
6. Reproduction All living things must produce offspring like themselves in order for their species to survive. This is the process known as reproduction. Plants produce seeds that give rise to new plants of thesame species. Animals lay eggs orhave babies. Reproduction can be of two types,
Sexual which involves two parents andthe union oftwo gametes andAsexual where oneparent can reproduce itself.
7. Nutrition Nutrition is needed for energy and growth, both plants andanimals need food.Plants are ableto make their own food by photosynthesis. They use sunlight to turn simple molecules like carbon dioxide and water into more complex carbohydrate molecules. Animals are unable to make theirown food sorely on other plants and other animals for their nutrition. Animals takein complex substances and break themdown into small,simple, soluble molecules whichcan be used for energy and growth
Nutrition: Energy required to carry out different life processes is obtained through the process of nutrition.Depending on themode of obtaining nutrition, organisms areclassified as autotrophs or heterotrophs. i. Autotrophs can prepare theirown food from simple inorganic sources such as carbon dioxide and water. Examples: Green plants and somebacteria. ii. Heterotrophs cannot synthesise their own food and are dependent on other organisms for obtaining complex organic substances for nutrition. Example: Animals and fungi
Autotrophic Nutrition: A type of nutrition in which organisms synthesize the organic materials they require frominorganic sources. Chiefsources of carbon and nitrogen arecarbon dioxide andnitrates, respectively. All green plants are autotrophic and use light as a source of energy for the synthesis of foodthrough photosynthesis.

The following events occur during this process. (i) Absorption oflight energy bychlorophyll (ii) Conversion of light energy to chemical energy and splitting of water molecules into hydrogen andoxygen. (iii) Reduction ofcarbon dioxide tocarbohydrates.
These greenplants absorbs waterfrom the soil by roots.Co2 enters fromthe atmosphere through stomata, Sunlight is absorbed by chlorophyll andother green parts of the plants.
Heterotrophic Nutrition: All heterotrophs depend on autotrophs fortheir nutrition. Thethree main typesof heterotrophic nutrition are:
1. Holozoic nutrition: Complex foodis taken intoa specialist digestive system and broken down into smallpieces to be absorbed. Eg: Ameoba, Humans 2. Saprophytic nutrition: Organisms feed ondead organic remains of other organisms. Eg: Fungi like bread moulds yeast andmushrooms.
Parasitic nutrition: Organisms obtain food fromother living organisms (the host), withthe host receiving no benefit fromthe parasite. Eg:cascuta, ticks, lice,leeches and tapeworms.
3. How doOrganisms Obtain Their Utrition?

In single celled organisms, the foodmay be taken in bythe entire surface. Eg: Amoeba takes in food using temporary finger-like extensions of the cell surface which fuseover the food particle forming a food-vacuole. Inside the food vacuole, complex substances are broken down into simpler ones which then diffuse into the cytoplasm. The remaining undigested material is moved to the surface of the cell andthrown out.
Nutrition in Human Beings: In humans, digestion of food takesplace in the alimentary canal, made up of various organsand glands.
In the mouth,food is crushed into small particles through chewing and mixedwith saliva, which contains amylase for digesting starch.
On swallowing, foodpasses through the pharynx and oesophagus to reach thestomach. Gastric juice contains pepsin (for digesting proteins), HCl and mucus.
The hydrochloric acidcreates an acidic medium which facilitates the action of the enzyme pepsin. The mucus protects the inner lining of the stomach from the action of the acid under normal conditions. From the stomach, the food now enters the small intestine. The small intestine is the siteof the complete digestion ofcarbohydrates, proteins and fats.
The liver secretes bilewhich emulsifies fat. The pancreas secretes pancreatic juice which contains the enzymes amylase, trypsin andlipase for digesting starch, proteins and fats, respectively. In the small intestine, carbohydrates, proteins and fats arecompletely digested intoglucose, aminoacids, fattyacids and glycerol. The villi of the small intestine absorb the digested foodand supply it to every cellof the body. The unabsorbed foodis sent intothe large intestine where more villi absorb water fromthis material. The rest of the material is removed fromthe body via the anus.
Respiration: During respiration, the digested foodmaterials are brokendown to release energy in the form of ATP. Depending on the requirement of oxygen, respiration maybe of twotypes:
i. Aerobic respiration: It occurs in the presence of air (oxygen).
ii. Anaerobic respiration: It occurs in the absence of (air) oxygen.
In all cases the first step is the break-down of glucose, a six-carbon molecule, into a three-caron molecule called pyruvate. This process taken place in thecytoplasm. Further, thepyruvate may beconverted into ethanol and carbon dioxide. This process takes place in yeast during fermentation. Since this process takes place in the absence of air (oxygen), it is called anaerobic respiration. Break-down ofpyruvate using oxygen takes place in the mitochondria. A large amount of energy isreleased in aerobic respiration as compared to anaerobic respiration. Some times when there is a lack of oxygen in our muscle cells, the pyruvate is converted into lactic acid. This build up of lactic acid in ourmuscles during sudden activity causes cramps.

Terrestrial organisms useatmospheric oxygen forrespiration, whereas aquatic organisms use oxygen dissolved in water.
In humans, inhalation of air occurs through the following pathway: Nostrils_ Nasal passage _ Pharynx _ Larynx _ Trachea _ Bronchus _ Bronchiole _ Alveolus (please put arrow marks——- à)

In human beings are is taken into the body through the nostrils. The air passing through the nostrils is filtered by fine hairs that line the passage. The passage is also lined with mucus which helps in this process. Fromhere, the air passes through the throat and into thelungs. Rings ofcartilage are present in the throat. These ensure that the air-passage does not collapse. Within the lungs the passage divides into smaller and smaller tubes which finally terminate in balloon-line structures which are called alveoli. The alveoli of lungs are richly supplied with blood and are the sites where exchange of gases (O2 and CO2) occurs between blood and the atmosphere. The blood brings carbon dioxide from the rest of the body for release into the alveoli, and the oxygen in the alveolar air is taken upby blood inthe alveolar blood vessels to be transported to all
the cells inthe body. During the breathing cycle, when air is taken in and let out,the lungs always contain a residual volume of air so that there is sufficient time for oxygen to be absorbed and for the carbon dioxide to be released. In humans, the respiratory pigment haemoglobin carries oxygen from the lungs to the different tissues of the body.This pigment in present in the redblood cells.
Transportation:
Transportation in Human Beings: The circulatory system is composed of the heart, blood and blood vessels which transport various materials throughout the body.
The heart:


The human heart has four chambers—two atria (right and left) and two ventricles (right and left). These chambers prevent the oxygen rich blood from mixing with the blood containing carbon dioxide. The right half of the heart receives deoxygenated blood, whereas the left half receives oxygenated blood.
The carbon dioxide –rich blood has to reach the lungs for the carbon dioxide to be removed, and the oxygenated blood from the lungs has to be brought back to the heart. This oxygen-rich blood isthen pumped to the restof the body. Ventricular walls are much thicker than atrial walls. Humans show double circulation i.e. blood goes through the heart twice and complete separation of oxygenated and deoxygenated blood. Arteries carry blood from the heart to different parts of the body, whereas veins deliver the blood back to the heart. Arteries are connected to veins by thin capillaries, wherein materials are exchanged between the bloodand cells. Blood has platelet cells which circulates around the body and prevent the blood loss at the site of injury. Lymph is also involved in transportation. It is similar to the plasma of blood but colourless and contains less protein. It drains into lymphatic capillaries from the intercellular spaces which join tofrom large lymph vessels that finally open intolarger veins. It carries digested and absorbed fat from intestine and drains excess fluid from extra cellular space back into the blood.
Transportation in plants: Plant transport systems will move energy stores from leaves and raw materials from roots. These two pathways are constructed as independently organized conducting tubes. One, the xylemmoves waterand minerals obtained from the soil.The other, phloem transports products ofphotosynthesis from the leaves where they are synthesised to other partsof the plant. The component of xylem tissue (tracheids and vessesls) of roots, stems, leaves are interconnected to form a continuous system of water conducting channels that reaches all parts of the plant. Transpiration creates a suction pressure, as a result of which water is forced into the xylem cells of the roots. Then there is a steady movement of water fromthe root xylem to all partsof the plant parts through theinterconnected water conducting channels. The loss ofwater in theform of vapour from the aerial parts of theplant is known as transpiration.
Thus it helps in the absorption and upward movement of water and minerals dissolved in it from rootsto the leaves. It also regulates temperature.
The transport of soluble products of photosynthesis is called translocation and itoccurs in phloem. It transports amino acids and other substances. The translocation of food and other substancestakes place in the sieve tubes with the help of adjacent companion cells both in upward and down ward directions. The translocation in phloem is achieved by utilising energy. Material like sucrose is transferred into phloem tissue using energy from ATP. This increases the osmotic pressure of the tissue causing water to move into it. This pressure. This allows the phloem to move material according to the plant’s needs. For example, in the spring, sugar stored in root or stem tissue would be transported to the buds which need energy to grow.
Excretion: During excretion, theharmful metabolic nitrogenous wastes generated areremoved from thebody
Excretion in Human Beings:

In humans, a pair of kidneys, a pair of ureters, the urinary bladder and the urethra constitute the excretory system. Kidneys are located in the addomen, one on either side of the backbone. Urine produced inthe kidneys passes through the ureters into the urinary bladder where it is stored until it is released through the urethra. Each kidney has large numbers of basic filtration units called nephrons. Some substances in the initial filtrate, such as glucose, amino acids, salts and a major amount of water, are selectively re-absorbed as the urine flows along the tube. The amount of water re-absorbed depends on how muchexcess water there is in the body, and on how muchof dissolved wastethere is tobe excreted. The urine forming in each kidney eventually enters a long tube, the ureter, which connects the kidneys with the urinary bladder until the pressure of the expanded bladder leads to the urge to pass it out through the urethra. The bladder is muscular so it is under nervous control. As a result wecan control theurge to urinate.
Excretion in plants: Plants do nothave an excretory system and carryout excretion in various wayssuch as transpiration, releasing wastes into the surrounding soil, losing their leaves and storing waste materials in cell vacuoles. Other waste products arestored as resins and gums in oldxylem.
Use the below buttons to explore our free services to find more…
Hindi Version Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term:
How do Organisms Reproduce | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Content Study Tools Content … Key Terms Topic Terminology Term: Important Tables Topic Terminology Term: Assessments Test Your Learning readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Content
Study Tools
Content …
Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term:
Heridity | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Content Study Tools Audio, Visual & Digital Content Chapter 9 -Heredity and Evolution 1. Accumulation of variation during Reproduction. Variations in an individual may be an advantage or disadvantage for it. It may enable or disable it to cope with changes in the environment. Advantageous variations… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Content
Study Tools
Audio, Visual & Digital Content
Chapter 9 -Heredity and Evolution
1. Accumulation of variation during Reproduction. Variations in an individual may be an advantage or disadvantage for it. It may enable or disable it to cope with changes in the environment. Advantageous variations are selected by environmental factors. For example bacteria that can withstand heat will survive better in a heat wave. Such heritable variations lead tothe evolution andformation of newspecies. An advantage of sexual reproduction is that the variations accumulated in the gametes of each sex are combined when they fuse to form the zygote. Hence an offspring produced from the zygote receives and carries the variations of both the parents. On the other hand, in asexual reproduction there are minor differences among the offspring. These are due to small errors in DNA copying. As gametes and zygote formation are not involves the asexually produced offspring arequite similar. Theyhave fewer variations accumulated over generations.
2. Heredity: The process ofpassing traits fromparent to offspring is called heredity. Trait is any characteristic thatis transferred fromparent to offspring. e.g. height and colour. 2.1 inherited traits. In humans, eye color is an example of an inherited characteristic: an individual might inherit the brown-eye trait from one of the parents. Inherited traits are controlled by genes and the complete set of genes within an organism’s genome is called itsgenotype. 2.2 Rules for theInheritance of traits- Mendel’s contributions: Gregor Johann Mendel was a pioneer among geneticists who put forward the concept of inheritance of characteristics or traits from parent to offspring. Mendel proposed the principle of inheritance and is known as the “Father of Genetics”. Mendel has chosen pea plants for his experimentation and found variations among them. Gene is a structural and functional unit of heredity and variations. Gene is a DNA segment on the chromosome. Genes control the expression of characteristics. Mendel called the genes to be factors. Traits can be either dominant or recessive. Tallness in a plant is a dominant trait, controlled by a dominant allele and is represented by “T” (capital). Shortness in a plant is a recessive trait, controlled by a recessive allele and is represented by “t” (small). · Homozygous is a condition in which a gene possesses a pair ofthe same alleles (TT or tt)for a single characteristic. · Heterozygous is a condition in which a gene possesses a pair of different alleles (Tt) fora single characteristic. Phenotype is a morphological expression of a single character. For example, tallness or shortness represents the phenotype of the plant. Genotype is the genetic make-up of a cell, an organism, or an individual (i.e. the specific allele make-up of the individual), usually with
reference to a specific characteristic under consideration. Alleles combine to make agenotype, such as TT or Tt or tt. Punnettsquare is a statistical method that was usedby Mendel to predict thepossible genotypes andphenotypes of the offspring.
Monohybrid inheritance It is the inheritance ofa single characteristic controlled by different alleles of thesame gene. · F1 generation is thefirst filial generation offspring produced by crossing twoparental strains. visible. All the progeny of F1 generation weretall i.e. thetraits of onlyone parent were · generation isthe second filial generation offspring produced by crossing F2 F1’s. TheF2 progeny were not all tall. Instead, one quarter of themwas short indicating both the traits – that oftallness and shortness were inherited inthe F2 plants. · Genotypic ratio – 1:2:1, Phenotypic ratio – 3:1. Dihybrid inheritance It is thesimultaneous inheritance oftwo characters. Dihybrid inheritance is theexperimentation of twocharacteristics with their four contrasting traits. For instance, dihybrid inheritance involves a plant producing round and yellow seeds (RR and YY) crossing with a plant producing wrinkled green seeds(rr and yy). · · F1 progeny produces roundand yellow seeds(R and r, and Y and y)in which roundand · yellow are dominant traits. F2 progeny were similar to their parents and produced roundyellow seeds, whilesome of · them produced wrinkled green seeds. However, some plants of the progeny even showed new combinations, like round-green seedsand wrinkled-yellow seeds. Thus the tall/ shorttrait and theround seed/wrinkled seedtrait are independently inherited. F2
2.3 How do thesetraits get expressed? A section of DNA that provides information for one protein is called the gene for that protein. The proteins synthesized according to this information may be enzymes that catalyse biochemical reactions. Each trait is the outcome of several suchbiochemical reactions eachof this is controlled by a specific enzyme. Each parent contributes one copy of the gene for a particular character. Thus there are two genes for every character. In the gamete, however, only one copy is present because of reduction division and these may be either maternal or paternal origin. When two germ cells combine they will restore the normal number of gene copies in the progeny ensuring the stability of theDNA of thespecies.
2.4 Sex determination It is a mechanism which determines the individual to be a male or a female based on the sex chromosomes present in it. In human beings, sex is determined by genetic inheritance. Genes inherited fromthe parents determine whether an offspring will be a boy or a girl.Gene for all
the characters are linearly arrange on the chromosomes. The chromosomes that carry genes for sexual characters are called autosomes or sex chromosomes while those that carry genes for the vegetative characters are called autosomes ornon sex chromosomes. Women have XX chromosomes whilemen have XY. All the children will inherit an X chromosome from their mother regardless of whether they are boys or girls. Thus the sex of the children will be determined by what they inherit from their father.
3. Evolution: All the life on Earth has descended from a common ancestor. Evolution is the sequence of gradual changes over millions of years in which new species are produced. Charles Robert Darwin was an English naturalist who observed various species of life on the earth and put forward the idea of “evolution of species by natural selection.” He said that a species inherits its characters from its ancestors. Acquired and inherited traits: An acquired trait is not transmitted to the off spring. In sexually reproducing organisms germ cells are produced in the reproductive organs, while the rest of the body has somatic cells. Changes in somatic cells due to environmental factors are not transmitted to the offspring. This is because a change in a somatic organ caused by a physiological response by the body does not bringabout a corresponding changein reproduction organs. A trait or character that is genetically inherited or passed down from generation to generation is known as inherited trait. Hugo de Vries explained the mechanism of heritable variations. According to him heritable variations arise when there is a change in the genes of the germplasm. He called it mutation. If a particular trait spreads in the population, it means that is favuored by natural selection.
4. Speciation: Speciescan be defined as a group of individuals ofthe same kindthat can interbreed and produce fertile progeny. Speciation: It is an eventthat splits a population into two independent species which cannotreproduce among them.
· Process of speciation-Genetic drift: It occurs due to changes in thefrequencies of particular genes by chance alone. e.g. If a hurricane strikes the mainland, and bananas with beetle eggs on themare washed away to an island. Thisis called a genetic drift.
· Process of speciation – natural selection: These are the variations caused in individuals due to natural selection which lead to the formation of a new species. e.g. If the ecological conditions are slightly different on the island as compared to the mainland, it leads to a change in the morphology and food preferences in the organisms over the course of generations.
Process of speciation -splitting of population: A population splits into different sub- populations due to geographical isolation thatleads to theformation of a new species.
Natural selection: It explains that organisms that are physiologically or behaviourally betteradapted for theenvironment are selected. Selected organisms can survive and reproduce.
Genetic drift: It is the genetic variation in smallpopulations caused by a specific environmental factor. Gene flow: It is the transfer of genes from one population to another due to migration. Breeding between the brown and green beetles introduces new gene combinations into the population. Over generations, genetic drift will accumulate different changes in each sub population. Also, natural selection may also operate differently in the different geographic locations. Speciation due to inbreeding, genetic drift and natural selection will be applicable to all sexually reproducing organism. 5. Evolution and Classification: Characteristics are the hereditary traits transmitted from parent organisms to their offspring. These are details of appearance or behavior in other words a particular form or a particular function. It shows how closely organisms are related with respect to evolution. The more characteristics two species will have in common, the more closely they are related. And the more closely they are related, the more recently they will have had a common ancestor. For example, a brother and a sister are closely related. They have common ancestors in the first generation before them, namely their parents. A girl and her first cousin are also related, but less than the girl and her brother. This is because cousins have common ancestors, their grandparents in the second generation before them, not in the first one. 5.1 Tracing Evolutionary relationships: Characteristics are of two types namely, homologous characteristics or analogous characteristics. · Homologous characteristics are organs that have the same basic structure and origin, but different functions. For example, mammals, birds, reptiles and amphibians have four limbs with the same basic limb layout because they have inherited the limbs from a common ancestor. These limbshave been modified to perform different functions. · Analogous characteristics are organs that have different structures and are of different origin, but perform same functions. For example, the design of the wings of bats and the wings of birds look similar because they have a common purpose – to fly.
5.2 Fossils:
Usually, when organisms die, their bodies will decompose and be lost. But sometime some body parts may not decompose completely and they will eventually harden and retain the impression of the body parts. All such preserved traces of living organisms are called fossils. Fossils are the remains or traces of a plant or animal that existed in a past geological age, and that has been excavated from the soil. Fossilisation is the process in which an organism is converted into a fossil. Paleontology is the study of fossils.
There are two ways to determine the age of fossils. One way is to dig the earth and start finding fossils. The second way of dating fossils is by detecting the ratios of different isotopes of the same element in the fossil material.
5.3 Evolution by Stages:
Evolution is a gradual process- no organism evolved suddenly. Complex organs evolved in organisms gradually. The eyes of the octopus and the eyes of vertebrates have evolved independently. These similarities of structure, despite of different origins provide a classic example of biological convergence. Biological convergence is a phenomenon by which two unrelated organisms become quite alike after a period of time through few generations, if it is assumed that they have a common ancestor. A change that is useful for one property to start with can become useful for quite a different function. Forexample, long feathers were considered to provide insulation in cold weather. Some reptiles like the dinosaur had feathers but very few were adapted for flying. In the present day, birds use feathers for flight, which is an example of adaptation. It is a characteristic of a particular animal may, post-evolution be useful for performing a totally different function. It is all very well to say that very dissimilar looking structures evolve from a common ancestral design. It is true that analysis of the organ structure in fossils allow us to make estimates of how far back evolutionary relationships go. The wild cabbage plant is a good example. Broccoli, kohlrabi and kale areproduced from itsancestor wild cabbage by artificial selection. Another way of tracing evolutionary relationships depends on the changes in DNA during reproduction. Comparing the DNA of different species should give us a direct estimate of how much the DNA has changed during the formation of new species. This method is now extensively used to define evolutionary relationships.
6. Evolution should not beequated with progress.
Evolution is simply generation of diversity and the shaping of the diversity by environmental selection. It is not as if the newlygenerated species arein any way better than the olderone. It is just natural selection and genetic drift have together led to the formation of a population that cannot reproduce with the original one, as in case of the evolution of humans and chimpanzees froma common ancestor. In evolution thenew forms evolved are more complex than their ancestors. It is theadaptability of a species to the environment that supports its survival not the complexity of the species. Each species, whether complex or simple is subjected to natural selection. Each species hasto go through the process of natural selection to survive andreproduce. In evolutionary terms, we cannot say that a particular species has a better design than another. Each species is well suited and adapted to its environment and hence is good enough to live andreproduce.
6.1 Human Evolution: The tools used to traceevolutionary relationships are excavation, time-dating, studying fossils, and determining DNA sequences have been usedfor studying human evolution. All the human beings in the world, whether they are African or American, share the same gene pool and hence all modern humans belong to the same species- Homo sapiens. There are, however, a large number of genes in the gene pool that serve as the source of individual variations. It is forthis reason that no two individuals are identical in looks, abilities, behavior, etc. therefore, there is great diversity in human features such as skin colour, height, hair colour, and so on. But there is no biological basis for assuming that humans with different features belong to different races.
Hindi Version Content …
Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term:
Control and Coordination | Study
Mind Map Overal Idea Content Speed Notes Quick Coverage Nervous system is the organ system present in the animals to control and coordinate different activities of the body. Nervous system comprises ofthe brain, thespinal cord, anda huge network of nerves thatare spread throughout the body. The nervous system is responsible for sending, receiving and processing… readmore
Mind Map
Overal Idea
Content
Speed Notes
Quick Coverage
Nervous system is the organ system present in the animals to control and coordinate different activities of the body.
Nervous system comprises ofthe brain, thespinal cord, anda huge network of nerves thatare spread throughout the body.
The nervous system is responsible for sending, receiving and processing messages in the form of chemical signals called as impulses. (Scroll down till end..)
Study Tools
Audio, Visual & Digital Content
Animals- Nervous System:
Nervous system is the organ system present in the animals to control and coordinate different activities of the body.
Nervous system comprises ofthe brain, thespinal cord, anda huge network of nerves thatare spread throughout the body.
The nervous system is responsible for sending, receiving and processing messages in the form of chemical signals called as impulses.
Nervous tissue is made up of an organized network of nerve cells or neurons.
It is specialized for conducting information via electrical impulses from one part of the body to another.
A neuron is the basic unit of the nervous system. Each neuron consists of three parts, namely, the cell body or cyton, branched projections called dendrites, the long process from the cell body, called the axon.
Synapse is a gap between two neurons.
Nerves are thread like structures emerging out of the brain and spinal cord.
Nerves branch out to all parts of the body and are responsible of carrying messages in the body.
Types of nerve cells or neurons:
- Sensory nerves send messages from the sense organs to the brain or spinal cord.
- Motor nerves carry messages back from the brain or spinal cord to all the muscles and glands in the body.
- Interneuron or relay neuron connects neuron within specific regions of the central nervous system. These are neither motor norsensory.
Reflex action:
What happens in reflex actions?
A reflex action, differently known as a reflex, is an involuntary and nearly instantaneous movement in response to a stimulus.
Reflex is an action generated by the body in response to the environment.
The process of detecting signal or the input and responding to it by an output action might be completed quickly. Such a connection is commonly called a reflex arc.
Reflex arcs are formed in the spinal cord itself; although the information input goes onto reach the brain.
In higher animals, most sensory neurons do not pass directly in to the brain, but synapse in the spinal cord.
Reflex arc continue to be more efficient for quick response.
Human brain:
Types of nervous system
The nervous system is divided into two systems as
- Central nervous system
- Peripheral nervous system.
Central nervous system:
Central nervous system includes the brain and the spinal cord.
It receives information from the body and sends out instructions to particular organs.
The brain has three such major parts or regions namely the fore brain, mid brain and hind brain.
Forebrain:
The forebrain is the main thinking part of the brain.
It consists of the cerebrum and diencephalon.
The cerebrum is the seat of memory and intelligence, and of sensory centres like hear, smell and sight.
The diencephalon is the seat for pressure and pain.
Midbrain:
The midbrain connects the forebrain to the hindbrain and controls the reflexes for sight and hearing.
Hindbrain:
The hindbrain consists of the cerebellum, pons and medulla.
The cerebellum coordinates muscular activities and maintains balance and posture.
The medulla controls involuntary activities like blood pressure, salivation, vomiting and heartbeat.
The spinal cord extends from the medulla of the brain through the whole length of the vertebral column and is protected by the vertebral column or backbone. Peripheral nervous system:
Peripheral nervous system consists of the cranial and spinal nerves arises from the brain and spinal cord respectively.
How are the tissues protected? Human brain is protected by the thick bones of the skull and a fluid called cerebrospinal fluid which provides further shock absorption.
How does the nervous tissue cause action? When a nerve impulse reaches the muscle the muscle fibre must move.
The muscle cells will move by changing their shape so that they shorten.
Muscle cells have special proteins that change both their shape and their arrangement in the cellin response to nervous electrical impulses.
When this happens new arrangements of these proteins give the muscle cells a shorter form.
Coordination in plants:
All living things respond to environmental stimuli.
Plants also respond to stimuli with the helpof chemical compounds secreted by thecells.
Plants being living organisms, exhibit some movements.
Plants show two different types of movements.
Types of movements shown by the plants are:
- dependent on growth
- independent of growth.
The plants also use electrical chemical means to convey this information from cellto cell but there is nospecialized tissue in plants for the conduction of information.
Plants respond to stimuli slowly by growing in a particular direction.
Because this growth is directional it appears as if the plant is moving.
Directional movements or Tropic movements:
Directional movements are also called as tropic movements.
- Directional movements movements can be either towards the stimulus or away from the stimulus.
- Positive phototropism is seen in shoots which respond by bending towards light.
- Negative geotropism is seen in shoots by growing away from the ground.
- Roots bend away from light exhibiting negative phototropism. They grow towards the ground exhibiting positive geotropism.
- Hydrotropism is a growth response in which thedirection is determined by the stimuli of water.
Chemotropism is a growth movement of a plant part in response to chemical stimulus.
e.g. Growth of pollen tubes towards ovules.
Hormones
Hormones are the chemical compounds released by stimulated cells.
Hormones diffuse all around the cell.
They are synthesised at places away from where they act and simply diffuse to the area of action.
Different plant hormones help to coordinate growth, development and responses to the environment.
Different hormones secreted by the plants are auxins, gibberellins, cytokinins, abscisic acid.
Auxins are the hormones synthesised at the tip of the stem. These help the plants in growth by cell elongation.
Auxin induces shoot apicaldominance.
Gibberellins are hormones that help in the growth of the stem, seed germination, bolting, and flowering.
Cytokinins are hormones present in the areas of rapid cell division, such as fruits and seeds.
They also promote the opening of the stomata.
Abscisic acid is a hormone that inhibits the growth in various parts.
It is also responsible for the closure of stomata. Its effects include wilting of leaves.
Hormones in Animals: Endocrine system is the system formed by ductless glands which secrete chemical substances called as hormones.
Endocrine glands release hormones directly in to the blood. Hormones are minute, chemical messengers thrown into blood to act on target organs.
Endocrine glands
Different types of endocrine glands present in our body are the pituitary gland, pineal gland, hypothalamus, thyroid, parathyroid, thymus, adrenal gland, pancreas, testes and ovary.
Adrenal glands:
These are located above kidneys.
Two regions of the adrenal gland are adrenal cortex and adrenal medulla.
• Adrenal cortex secretes the hormones like cortisol, aldosterone and androgens.
• Adrenal medulla secretes the hormones like adrenaline andnoradrenaline.
Adrenaline is also called the “hormone of fight or flight,” or the emergency hormone.
It prepares the body to face an emergency condition of physical stress, like danger, anger and excitement.
Thyroid gland:
• It is located in the neck, ventral to thelarynx. • It is the one of the largest endocrine glands. • The principal hormones produced by this gland are triiodothyronine and thyroxine.
• Thyroxine is a hormone that regulates the metabolism of carbohydrates, proteins and fats in the body.
Iodine is essential for the synthesis of thyroxin.
Deficiency of iodine in food causes goiter.
One of the symptoms of this disease is a swollen neck.
The pituitary gland:
• It is located at the base of the brain. • It is considered to be master gland as it secretes many hormones to regulate organs as wellas the other glands. • Different hormones secreted by this gland include Growth hormone, TSH, FSH, LH, ACTH, MSH, Vasopressin and Oxytocin.
Growth hormone regulates growth and development of the body. If there is a deficiency of this hormone in childhood, it leads to dwarfism.
Excess secretion of this hormone leads to gigantism.
Gonads:
Two types of gonads present in human beings are female gonads and male gonads.
Female gonads
• A pair of ovaries forms the gonads in female. • Ovaries are the female sex organs that lie one on either side of the abdominal cavity.
Ovaries produce two hormones, namely, oestrogen and progesterone. • Oestrogen controls the changes that occur during puberty, like feminine voice, soft skin and development in mammary glands. • Progesterone controls the uterine changes in the menstrual cycle, and helps in the maintenance of pregnancy.
Male gonads
• A pair of testes forms the gonads in males. • A pair of testes isthe male sexorgan located inthe scrotum, whichis outside theabdomen. • Testes produce the hormone testosterone. • Testosterone controls the changes, whichoccur during puberty, like deeper voice, development of penis, facial and bodyhair.
Pancreas: It is located just below the stomach within the curve of the duodenum. It is both exocrine and endocrine in function. • It secretes hormones such as insulin, glucagon, somatostatin and pancreatic polypeptide. • Insulin regulates the sugar level inour blood.
Insulin secreted in small amounts increases the sugar level in our blood which in turn causes a disease called diabetes mellitus.
Pineal gland: • It is located near the centre of the brain, dorsal to the diencephalon. • It produces the hormone melatonin. • Melatonin affects reproductive development, modulation of wake and sleep patterns, and seasonal functions.
Hypothalamus: • It is a neuro-endocrine part of the brain. • It links the nervous system and the endocrine system through the pituitary gland. • Hormones likeStomatostatin, Dopamine aresecreted by thisgland.
Parathyroid glands:
• These are two pairs of small, oval-shaped glands embedded on the dorsal surface of the thyroid gland present in theneck. • They secrete parathormone.
parathormone helps in regulation of calcium and phosphate ions inthe bones and blood. • Hypo secretion leads to tetany and hypersecretion causes osteoporosis.
Thymus gland:
• It is located infront of the heart, in the upper part ofthe sternum. • It produces the hormone thymosine. • It helps in the maturation of T-lymphocytes.
The timing and amount of hormones released are regulated by feedback mechanisms.
For example, if the sugar levels in blood rise, they are detected by the cells of pancreas which respond by producing more insulin.
As the blood sugar level falls, insulin secretion is reduced.
Hindi Version Key Terms
Topic Terminology
Term:
Important Tables
Topic Terminology
Term: